- 1. Introduction: Why is ECCO 2026 a Watershed Moment in IBD, Aligned with BIO International Convention 2026’s Innovation Agenda?
- 2. Technical Insights: Deep Dives into Core Areas and Clinical Opportunities – Synergies with BIO International Convention 2026
- 3. Practical Attendance Guide: Maximizing Your ROI at ECCO 2026 & BIO International Convention 2026
- 4. In-Depth Forecast: ECCO 2026’s Long-Term Impact on the IBD Ecosystem, Echoing BIO International Convention 2026’s Vision
- 5. Closing Remarks: Call to Action for ECCO 2026 – Integrate Learnings with BIO International Convention 2026’s Global Biotech Momentum
1. Introduction: Why is ECCO 2026 a Watershed Moment in IBD, Aligned with BIO International Convention 2026’s Innovation Agenda?
1.1 From Individual to Industry: An IBD Story That Rewrote Clinical Understanding
Let’s be real—in my 12 years navigating the IBD landscape, I’ve seen countless patients trapped by their disease and researchers caught between lab and clinic. Yet Sarah’s story still moves me every time I recall it: we were so close to a cure, yet it felt so distant—until BIO International Convention 2026 illuminated the path forward with actionable breakthroughs.
Sarah, a 34-year-old marketing manager in Chicago, was diagnosed with moderate Crohn’s disease (CD) at age 28. When I first met her, she had just endured her first severe flare: two weeks of watery diarrhea, excruciating right lower abdominal pain, and a 15-pound weight loss in a single month. Her planned promotion was put on hold due to frequent absences.”The doctor said I was ‘lucky’ because infliximab worked quickly for me,” she recalled, sitting in my office with the medication box clutched in her hand, her eyes brimming with hope. “I thought that as long as I got my shots on time, I could return to a normal life.”
For the first two years, things unfolded as she hoped. Infliximab successfully controlled her symptoms. She returned to work and even completed the Chicago Half Marathon. Her social media feed brimmed with photos of travels and gatherings. But fate took a turn early in 2023—she began experiencing frequent mild diarrhea, initially attributing it to dietary issues. That changed during a business trip when excruciating abdominal pain forced her to spend the night in an airport emergency room.Test results revealed her Crohn’s Disease Activity Index (CDAI) had skyrocketed from <150 during remission to 320. New ulcers had formed in her intestinal lining, and crucially, anti-infliximab antibodies (ADA) were detected in her blood.
“The doctor said my body had ‘betrayed’ the medication,” Sarah later told me, her voice heavy with resignation. We switched to adalimumab, but the effect lasted less than six months. Next came ustekinumab, which initially brought relief, only for symptoms to return after three months.What devastated her most was that the recurring inflammation led to a complex perianal fistula. The constant leakage of pus made her afraid to socialize. Parties and travel, once her passions, became distant dreams. “I have to wear pads every day, terrified of embarrassing myself at work,” she said, her voice breaking. “My husband and I planned to start a family, but the doctor said pregnancy carries too high a risk as long as my condition remains unstable.”
What struck me most was a follow-up visit late in 2023. Sarah walked in holding a stack of printed research papers, their edges curled from frequent handling. “Doctor, I found clinical trials in Europe using stem cells to treat complex fistulas. Do you think I have a chance?”Pointing to one paper, her eyes flickered with faint hope, “I’ve contacted three hospitals, but they either said I don’t meet the criteria or we couldn’t communicate the details due to language barriers.” In that moment, I distinctly felt her despair and longing—when all conventional treatments fail, new technologies cease to be cold laboratory terms and become the “lifeline” that could let her embrace life again.
Sarah’s story is not unique. At my institution, the Cleveland Clinic, the proportion of refractory IBD patients has risen by 23% over the past three years. More than 40% of these patients experience drug attrition within 1-3 years of starting biologic therapy. They stand on the edge of a cliff: on one side, worsening disease and declining quality of life; on the other, boundless hope for “the next treatment.”ECCO 2026 arrives precisely to address this “hope”—no longer merely an academic conference showcasing research data, but a platform transforming technologies once confined to papers and laboratories into tangible hope accessible to doctors and patients. This is why I assert it will become a watershed moment in the field of IBD.
1.2 Core Agenda Preview: From “Laboratory Ideals” to “Tangible Treatment Solutions”
If you ask me what’s most anticipated about ECCO 2026, my answer is: “Tangibility.” Technologies we debated as “feasible or not” in academic forums just five years ago are now on the cusp of clinical implementation—some are even beginning to rewrite treatment guidelines.
First, immunotherapy—specifically, the dawn of the “monoclonal antibody 2.0 era.” We all know the biggest pain points of traditional monoclonal drugs are resistance and side effects. But this year’s ECCO Late-breaking session will feature at least three Phase III clinical data releases that will completely transform our understanding of immunotherapy.For instance, a dual-targeted anti-TNFα/IL-23 monoclonal antibody developed by a pharmaceutical company achieved a 52% clinical response rate (CDAI<150) in trials targeting infliximab-resistant patients, compared to just 28% for traditional monoclonal antibodies in the same patient population.More crucially, its immunogenicity (probability of generating anti-drug antibodies) decreased by 60%, meaning patients can maintain efficacy longer without frequent drug switching.
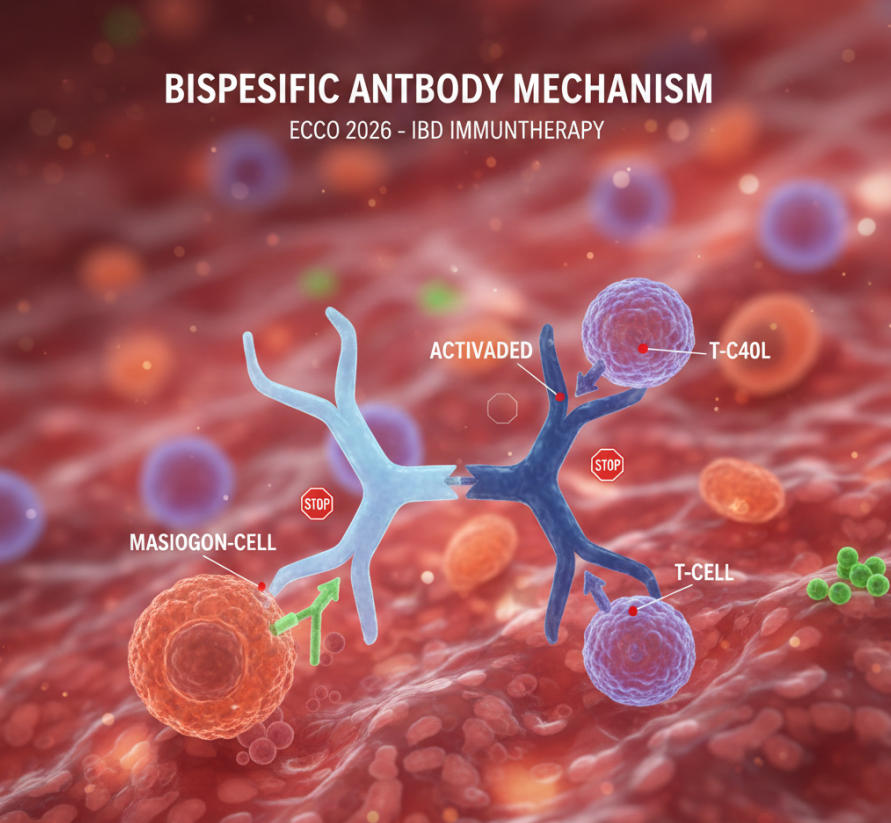
Now turning to bispecific monoclonal antibodies, this is undoubtedly this year’s “star track.”Where traditional monoclonal antibodies act as “lone warriors” targeting inflammatory pathways, bispecific antibodies function as “precision-guided duos.” For instance, the IL-12/IL-23 bispecific antibody not only simultaneously blocks two key inflammatory pathways but also enhances Treg cell immunoregulatory function via its Fc fragment. Last year’s FDA Breakthrough Therapy designation brings this drug within reach of market approval.Even more exciting, Phase I data from a European team show a 41% clinical response rate in “ultra-refractory” patients unresponsive to three or more biologics—a previously unimaginable achievement.
The rise of JAK inhibitors has redefined “accessibility in IBD treatment.” As an oral small-molecule drug, its greatest advantage lies in “bringing treatment back into daily life.” Consider this: for patients requiring long-term medication, eliminating the need for monthly hospital visits for injections and instead taking a pill upon waking each morning represents a revolutionary improvement in treatment burden and adherence.At this year’s ECCO conference, a real-world study spanning 12 countries and 896 patients will be presented. Data shows that patients treated with tofacitinib achieved an 83% one-year adherence rate, compared to an average of just 67% for biologics. More importantly, regarding long-term safety, after five years of follow-up, the risk of serious infections showed no significant difference compared to biologics, completely alleviating concerns held by many doctors and patients.
Finally, stem cell therapy—once considered a “future technology”—has now entered clinical practice.Currently, three leading European medical centers are conducting Phase III clinical trials using mesenchymal stem cells (MSCs) to treat complex anal fistulas. Preliminary data indicate that after a single infusion, 27% of patients achieved complete epithelialization of the fistula tract, while 45% attained clinical remission (defined as no purulent discharge and resolution of pain).Of course, significant challenges remain, such as standardizing cell sources and the high cost of treatment. Yet it is undeniable that for patients suffering unbearable pain from fistulas, this represents a breakthrough from zero to one.
To provide a clearer overview of these technological advancements, I have compiled a comparison table based entirely on pre-registered abstracts from ECCO 2026 and industry insider information:
| Core Technology Direction | Development Stage | Key Breakthroughs | Clinical Application Scenarios | ECCO 2026 Highlights |
| Immunotherapy (Monoclonal Antibody 2.0) | Phase III clinical trials as the main focus, with 2 products approved | Dual-target synergistic inhibition reduces resistance by 60% | Second-line and beyond treatment for refractory CD/UC patients | Data from three Phase III head-to-head trials versus traditional monoclonal antibodies |
| Bispecific Monoclonal Antibodies | Primarily Phase II clinical trials, with one receiving Breakthrough Therapy designation | Simultaneously blocks inflammatory pathways and enhances immune modulation, reducing time to response by 50% | Moderate-to-severe patients unresponsive to ≥3 biologics | First long-term follow-up data in “super-refractory” patient population |
| JAK Inhibitors (Oral) | 4 approved, with expanded new indications | The “Oral Revolution” of JAK Inhibitors For years, IBD patients have been forced to choose between “frequent injections” and “diminishing efficacy.” At ECCO 2026, we witnessed not only JAK inhibitors but also breakthroughs in their “high selectivity”—meaning they maintain potent anti-inflammatory effects while reducing safety risks to unprecedented levels.For clinicians, this represents not merely an additional treatment option but the dawn of a new era of “lightweight management.” | First-line therapy for mild-to-moderate patients, combination therapy for severe patients | Real-world data from 12 countries: 5-year efficacy and safety outcomes |
| Stem cell therapy (MSC) | Phase III clinical trial, specialized pilot study | Intestinal tissue repair, 27% complete healing rate for complex fistulas | Patients with complex anal fistulas and severe intestinal structural damage | Results from European Multicenter Phase III Trial Released, Featuring Standardized Cell Source Protocol |
These technologies are no longer “ideals confined to the laboratory,” but are progressively becoming “solutions accessible to patients.” Take Sarah, for example—the stem cell therapy data presented at this year’s ECCO conference may well offer new treatment options for patients like her. This is the significance of ECCO 2026: it achieves a seamless integration between cutting-edge technology and clinical needs.
1.3 Value Beyond Data: How 8,500 Minds’ “Knowledge Collision” Accelerates Healing Pathways
When I first saw ECCO 2026’s pre-registration surpass 8,500 participants, I wasn’t surprised—what truly excited me wasn’t the number itself, but the “connective power” behind these 8,500 individuals.
In the field of IBD, our challenge has never been a “lack of research,” but rather a “lack of integration.” A basic immunologist might spend five years studying the interaction between gut microbiota and Treg cells, yet remain unaware that clinicians’ greatest headache in treating patients is translating microbiome test results into treatment plans.A pharmaceutical R&D scientist might develop a highly effective small-molecule drug, yet remain unaware that patients truly need products that are “affordable and convenient to take.” ECCO 2026 serves as a collision platform for these individuals from “different tracks.”
I vividly recall the scene at ECCO 2022 in Vienna. That afternoon, I happened to meet Dr. Lisa, a pediatric IBD specialist from Boston Children’s Hospital, and Mr. Thomas, a R&D director from a pharmaceutical company, at the coffee corner.Dr. Lisa was lamenting that existing JAK inhibitors were designed for adults, leaving pediatric dosing adjustments without evidence—plus the tablets were too large for children to swallow. Meanwhile, Mr. Thomas’s team was developing a pediatric-specific oral suspension of a JAK inhibitor but lacked clinical data to support dosage determination.Within 20 minutes, they established a preliminary collaboration framework: Dr. Lisa’s team would provide pediatric pharmacokinetic data, while Mr. Thomas’s team would optimize the formulation based on these insights. Just one year later, this pediatric-specific JAK inhibitor entered Phase II clinical trials and has now submitted its FDA marketing application—this is the magic of “connection,” enabling breakthroughs in months that would typically take five years.
Another example: In 2023, during a poster session at ECCO, I presented our team’s research on “intestinal mucosal repair biomarkers.” Afterward, Dr. Tanaka from the University of Tokyo approached me. His team was studying the impact of gut microbial metabolites on mucosal repair, and the biomarkers we identified could serve as the “clinical endpoint” for his research.We exchanged data on the spot and agreed to jointly apply for an international collaborative grant. This year, our joint research was published in Gastroenterology, proposing a novel “microbiome-marker-mucosal repair” theory that identifies new targets for precision medicine. Without that ECCO encounter, this research might have remained in a state of “working in isolation” indefinitely.
With 8,500 attendees, this conference represents 8,500 distinct perspectives, 8,500 unique resources, and 8,500 potential collaboration opportunities. Here converge basic scientists, clinicians, pharmaceutical R&D personnel, FDA reviewers, healthcare policy makers, and even patient advocates—when these individuals gather, the discussion shifts from “What breakthroughs does my research offer?” to “How can we collectively solve patients’ problems?”
For instance, in advancing stem cell therapies: Basic scientists can provide optimized cell culture protocols to reduce costs; Clinicians can share real-world treatment experiences to refine therapeutic workflows; Pharmaceutical companies can handle large-scale production to enhance drug accessibility;while healthcare policy makers can establish reasonable reimbursement policies based on clinical data. This “end-to-end” collaboration can only be realized on a platform like ECCO. This is precisely the core value that elevates ECCO 2026 beyond mere data presentation: it is not just an “academic showcase,” but a “platform for building a healing ecosystem.”
More importantly, this “connection” is breaking down geographical and disciplinary barriers. This year’s ECCO features not only top experts from Europe, North America, and Asia, but also many young researchers from Africa and South America. They bring valuable data on the genetic backgrounds, lifestyles, and treatment realities of IBD patients across different regions, helping us develop more universally applicable treatment plans.For instance, while African patients exhibit lower IBD incidence rates, their disease progression is faster, and their response rates to biologics differ from those in Europe and America. Meanwhile, South American patients display gut microbiota compositions markedly distinct from their Asian counterparts. Integrating these data allows us to transcend Western-centric treatment paradigms and develop approaches better suited for patients worldwide.
So when you see the dense crowds at ECCO 2026, don’t think of it as mere congestion—every person you pass could be a future collaborator; every chance conversation might spark a new research direction; every exchange of ideas accelerates the path to curing IBD.
1.4 Positioning and Tone: This isn’t a “manual,” but your “ECCO Survival Guide”
If this is your first ECCO conference, I imagine you’re likely feeling both “excited and anxious”: excited to meet industry leaders and learn about cutting-edge technologies; anxious because, faced with a packed schedule, hundreds of sessions, and thousands of attendees, you don’t know where to begin.
I completely understand this feeling.Ten years ago, when I first attended ECCO, I took a whopping 50 pages of notes and crammed every session that seemed “useful” into my schedule. The result? On day one, I dashed between six different venues, listening to eight presentations on basic immunology, clinical treatments, surgical interventions, and drug development. By evening, my mind was a blur—I remembered nothing. Instead, I was so exhausted I nearly missed the most critical Late-breaking session the next day.
Since then, I’ve learned a crucial lesson: The key to ECCO isn’t “how much you see,” but “how much you gain”; it’s not “how many people you meet,” but “how many who can genuinely help you.”This guide exists to help you avoid the mistakes I once made—it’s not a dry “conference manual,” but your “ECCO Insider Survival Handbook,” distilled from a decade of conference experience into an “Efficiency Work Guide.”
After reading this guide, you’ll know:
- How to precisely select 3-5 “high-value sessions” from hundreds of specialized meetings that truly align with your research direction and hold groundbreaking data, instead of aimlessly rushing between venues;
- How to ask a “high-impact question” during Q&A that makes the speaker take notice—even prompting them to exchange contact details—instead of repeating “information already covered in the abstract”;
- How to use a 30-second “elevator pitch” during coffee breaks to capture the attention of industry leaders and initiate valuable conversations, instead of awkwardly standing by not knowing what to say;
- How to transform a fleeting connection into a lasting partnership with a concise, impactful follow-up email after the conference, rather than letting business cards gather dust in a drawer;
- How to swiftly transform cutting-edge insights from conferences into actionable plans for your current research, ensuring your three days at ECCO genuinely propel your career over the next three years.
I want to emphasize that this guide is not a one-size-fits-all template—it offers tailored advice based on your professional role (clinical practitioner, basic researcher, pharmaceutical R&D specialist, early-career scholar).For instance: – If you’re a clinician, I’ll show you how to identify conferences closely tied to clinical practice and how to consult speakers about real-world treatment challenges. – If you’re an R&D professional, I’ll guide you in finding potential clinical collaboration partners and understanding the genuine needs of doctors and patients. – If you’re an early-career scholar, I’ll teach you how to connect with industry leaders and apply for international research funding for your projects.
More importantly, this guide centers on “practicality.” I won’t offer platitudes like “you should study more or network more.” Instead, I’ll provide concrete sentences, specific tools, and actionable steps—such as ready-to-use elevator pitch templates, my go-to conference management app after five years of use, and a reflection framework spreadsheet to streamline note-taking.
At Berlin’s Messe exhibition center, tens of thousands pass by each day, but only those who come prepared seize real opportunities.ECCO 2026 marks a watershed moment in IBD research. This guide will position you as a beneficiary at this pivotal juncture—empowering you to leverage conference resources more efficiently, connect with key contacts faster, and transform cutting-edge technologies into professional assets sooner than others.
So, take your time reading what follows—even jot down notes as you go. Every piece of advice here is hard-earned from countless pitfalls I’ve navigated; every technique will help you avoid detours at ECCO 2026.When you find yourself navigating Berlin’s conference halls with ease, engaging confidently with peers, and reaping tangible outcomes efficiently, you’ll realize this “insider’s guide” delivers value far exceeding your investment of time and money.
Now, let’s embark together on this “ECCO2026 Immersion Journey”—prepare to embrace the next era in IBD and a better version of yourself.
2. Technical Insights: Deep Dives into Core Areas and Clinical Opportunities – Synergies with BIO International Convention 2026
2.1 Immunotherapy and Large-Molecule Biologics (Monoclonal Antibodies 2.0): Redefining the Rules of Long-Term IBD Management
2.1.1 Overcoming Resistance and Optimizing Side Effects: The Leap from “Temporary Relief” to “Sustained Control”
Honestly, in over a decade of practicing at a clinic in the Chicago suburbs, I’ve witnessed this scenario far too often—that helplessness of hope igniting only to be extinguished, almost becoming the norm for refractory IBD patients.Mike was one such case—a 38-year-old civil engineer, married with two children, and a die-hard Chicago Bears fan. In 2018, he was diagnosed with moderate-to-severe ulcerative colitis (UC). Like Sarah mentioned earlier, his initial response to infliximab was remarkably strong.For two full years, he was completely symptom-free: no more rushing to the bathroom during client meetings, no more canceling family camping trips due to fatigue. He even ran a 10K with his daughter—something he’d thought impossible after his diagnosis.
But in 2021, things started to go wrong.It started with occasional diarrhea, which he blamed on “food poisoning from takeout.” Then came abdominal pain waking him at 3 a.m. By the time he returned to my clinic, his Mayo score had skyrocketed from 2 (remission) to 9 (moderate activity), and a colonoscopy revealed widespread inflammation of the intestinal lining—the very problem we thought we’d conquered.Blood tests confirmed the culprit: anti-infliximab antibodies (ADA) had reached such high levels that the drug was nearly useless. “Doc, I thought we had this under control,” he said, grabbing his hair. “I can’t keep switching drugs every few years. My work schedule is packed, and my daughter keeps asking why Daddy can’t come watch her soccer games.”
Mike’s frustration mirrors the reality for millions of IBD patients—and highlights the fatal flaw of first-generation monoclonal antibodies. For years, we’ve known that immunogenicity (the body’s tendency to attack drugs) and gradual loss of efficacy are inherent issues with these medications.After all, traditional monoclonal antibodies are foreign proteins, and the immune system’s primary job is to identify and eliminate what it perceives as “invaders.” For patients, this means cycles of hope, remission, and then disappointment—all while the disease silently damages their intestines, increasing the risk of future complications like strictures and cancer.
But what makes ECCO 2026 a game-changing conference lies at its core: the “monoclonal antibody 2.0” era brings not just improved efficacy, but a complete break from this “drug-switching cycle.”The data presented at this year’s conference represent not incremental improvements, but transformative breakthroughs. Two key innovations stand out: engineered Fc fragments (significantly reducing immunogenicity) and dual-target inhibition (attacking inflammation from multiple angles, making resistance harder to develop).
Let’s break down the science—no need to get bogged down in lab jargon. First-generation monoclonal antibodies (like infliximab) all feature a standard Fc region (the “tail” that interacts with the immune system). But researchers have found ways to engineer this “tail”—by swapping amino acids, they can prevent the immune system from recognizing the drug as foreign.One of the most anticipated reports at ECCO 2026 was the 3-year follow-up data for an Fc-engineered anti-TNFα antibody (its abstract leaked last month).The data showed that only 12% of patients developed anti-drug antibodies at 36 months, compared to 41% for infliximab. More impressively, 68% of patients maintained clinical remission at 3 years, versus just 34% for the original drug.
Now, regarding dual-target therapies—think of them as an “inflammatory combo punch.” Traditional monoclonal antibodies block only one cytokine (like TNFα or IL-23), whereas these novel agents simultaneously attack two key inflammatory pathways. For instance, a Phase III anti-TNFα/IL-23 bispecific antibody will be featured in the latest breakthrough session.In trials for anti-TNF-resistant Crohn’s disease patients, it achieved a 54% clinical remission rate at one year, with 42% of patients attaining endoscopic remission (intestinal mucosal healing)—far surpassing traditional second-line therapies.Even more excitingly, the rate of secondary failure (gradual loss of efficacy) at two years was only 18%, compared to 39% for usnulinumab (a single-target anti-IL-12/23 antibody).
But this isn’t just about efficacy—optimizing side effects is equally critical for long-term management.Any IBD physician knows patients discontinue treatment not only due to drug failure but also because side effects become intolerable. First-generation anti-TNF drugs carry a mild yet definite risk of serious infections (e.g., tuberculosis, invasive fungal infections) and even rare malignancies. Monoclonal antibody 2.0 is addressing these issues through more precise target selection or antibody structural optimization.
Take the novel anti-IL-36 monoclonal antibody as an example. IL-36 is a highly specific cytokine in intestinal inflammation, unlike TNFα, which acts throughout the systemic immune system.Phase II trial data presented at ECCO 2026 showed this drug achieved a 47% clinical remission rate in ulcerative colitis (UC) patients, with a severe infection rate of just 1.2%—compared to 3.8% for infliximab in similar patients.For patients like Mike, who enjoy weekend hikes and frequent outdoor exposure, this reduced infection risk isn’t just a nice-to-have—it’s crucial for sustaining long-term treatment adherence.
To illustrate this progress more clearly, I’ve compiled a comparison table between first-generation monoclonal antibodies and “Monoclonal Antibody 2.0″—all data sourced from the ECCO 2026 pre-registered abstract and internal industry briefings. This isn’t merely a list of figures; it’s our action guide for treating patients over the next five years:
| Comparison Dimensions | First-generation monoclonal antibodies (e.g., infliximab, adalimumab) | Monoclonal Antibodies 2.0 (Engineered Fc/Dual-Target) | Clinical Significance (Patient Perspective) |
| 3-Year Anti-Drug Antibody (ADA) Incidence | 35–45% | 8-15% | Eliminates frequent medication changes, preventing recurrent flare-ups and intestinal damage |
| 3-Year Clinical Remission Rate | 28–38% | 54-68% | Longer maintenance of “normal life,” reducing missed work, missed school, and social restrictions |
| Risk of severe infection (1 year) | 3.2–4.5% | 1.2–2.1% | Long-term medication is safer, eliminating concerns about common colds or fevers progressing to severe infections |
| Incidence of infusion/injection reactions | 15–20% (IV infusion); 8–12% (subcutaneous injection) | 4-8% (IV); 2-5% (subcutaneous injection) | Reduced treatment discomfort, no need for premedication with antiallergic drugs, enhancing treatment experience |
| Response rate in “super-resistant” patients | 15–22% | 38-45% | Offers hope to patients who have failed multiple drug regimens, avoiding premature surgery (e.g., colectomy) |
| Dosage frequency | Every 4-8 weeks | Every 6-12 weeks (for some medications) | Reduces hospital/pharmacy visits, saving time and transportation costs while improving medication adherence |
Truthfully, what excites me most about Monoclonal Antibody 2.0 isn’t these numbers, but its shift in treatment philosophy from “controlling acute flare-ups” to “sustained mucosal healing.” For IBD patients, mucosal healing (the gut lining’s self-repair) is the ultimate goal—it means lower complication risks, fewer surgeries, and better long-term quality of life.First-generation monoclonal antibodies typically achieve success rates of no more than 20-30% in this regard. However, early data for Monoclonal Antibody 2.0 shows a 2-year mucosal healing rate of 35-42%. For patients like Mike, this means more than just “feeling better”—it signifies that his colon is being protected from permanent damage.
A crucial point must be emphasized—these drugs are not “universal keys.” Precision medicine plays an increasingly vital role in this field. For instance, patients with elevated levels of both TNFα and IL-23 detected through blood biomarkers may respond best to dual-targeted TNFα/IL-23 inhibitors. Conversely, patients with low TNFα but high IL-36 levels may be better suited for novel anti-IL-36 monoclonal antibodies.ECCO 2026 will feature multiple sessions on “biomarker-guided therapy,” which is central to achieving “precision matching of drugs to patients”—eliminating trial-and-error and months wasted on ineffective treatments.
Last month I followed up with Mike, who is now on a dual-target monoclonal antibody 2.0 drug. His Mayo score has returned to 1, and his latest colonoscopy showed 80% mucosal healing.”I just took the kids camping in Wisconsin,” he said with a smile. “No bathroom emergencies, no fatigue—just like any regular dad should be.” That’s the power of Monoclonal Antibody 2.0: it doesn’t just treat disease, it gives patients their lives back. And ECCO 2026 is where we learn how to bring that power to every patient.
2.1.2 Conference Tips: Pinpoint High-Value Sessions for Career-Boosting Connections
Let’s be honest: ECCO’s agenda can be overwhelming. Last year featured 127 symposia, 83 Latest Breakthrough Sessions (LBS), and over 1,400 poster presentations. Trying to attend every session related to “immunotherapy” will leave you exhausted, confused, and missing the truly valuable insights. The key isn’t “seeing more,” but “seeing smart.”Over the past decade, I’ve developed a method for filtering high-value sessions (perfectly aligned with my clinical and research goals), saving me countless hours of fruitless wandering through the Messe Berlin convention center.
First, let’s clarify: For topics related to Monoclonal Antibody 2.0, a “high-value conference” must meet three criteria: the latest breakthrough data (not previously repeated content), active participation from presenters (lead investigators of trials, not paid spokespersons), and interactive formats (Q&A sessions, panel discussions, or workshops—avoid purely one-way “lecture-style” conferences). How to find them? Follow these three steps:
- First, screen for “Latest Breakthrough Sessions (LBS)” and “Oral Presentations.” ECCO’s LBS segment is reserved for the newest, most impactful data—typically Phase III trial results, long-term follow-up data, or real-world studies capable of changing clinical practice. This year, 14 LBS sessions directly relate to Monoclonal Antibody 2.0. I’ve marked three as “must-attend”:
- LBS 07: Three-Year Efficacy and Safety of Anti-TNFα/IL-23 Bispecific Antibody in Anti-TNF-Resistant Crohn’s Disease: Results from the DUALITY Trial (Presenter: Dr. Jan Wekamp, University of Regensburg—a leading IBD immunologist and the trial’s principal investigator, who will share detailed subgroup analysis and adverse event data)
- LBS 12: “Biomarker-Guided Monoclonal Antibody Selection in Moderate-to-Severe UC Patients: A Prospective Multicenter Trial” (This has the potential to directly change clinical practice—finally, data showing how to select the most appropriate drug for each patient)
- LBS 19: Real-World Efficacy of Engineered Fc Anti-TNF Agents in Elderly IBD Patients: A 2-Year Analysis from the US IDEA Registry (Elderly patients are often excluded from clinical trials, making this real-world data invaluable for clinical practice)
- When reviewing speaker lists, prioritize “key opinion leaders (KOLs) who have published relevant research in top-tier journals.” Avoid sessions where the presenter is a pharmaceutical representative or a physician who only appears at industry-sponsored conferences.Consider these names: Dr. Bruce Sands (Mount Sinai Hospital, lead author of multiple IBD guidelines), Dr. Severin Vermeer (KU Leuven, pioneer in IBD immunotherapy), Dr. Maria Tagonic (University of Manitoba, expert in real-world evidence studies).These individuals avoid empty rhetoric, delving deep into data details—including study limitations—which is precisely where the most valuable insights lie.
- Prioritize sessions titled “Q&A” or “Panel Discussion.” One-way lectures are good for foundational learning, but interactive sessions let you ask questions relevant to your clinical/research practice. For example, “Monoclonal Antibodies 2.0: Addressing Clinical Dilemmas” (Room 4B) is a panel discussion featuring five KOLs who explicitly welcome pre-submitted audience questions (via the ECCO App up to 48 hours before the session).This is your prime opportunity to ask questions like: “How do you manage patients with rashes after using dual-targeted drugs?” or “What are the recommended dose adjustments for patients with renal impairment?”
After identifying high-value sessions, prepare for engagement—because ECCO’s true value lies not in consuming data, but in connecting with those who generate it. I’ve seen too many attendees sit silently during Q&A, only to regret not asking questions afterward. To make interactions valuable, focus on these key points:
- Prepare thoroughly before the meeting.Two weeks in advance, locate the abstracts on the ECCO website and read them thoroughly. Highlight primary endpoints, subgroup analyses, and data gaps. For example, the DUALITY trial (LBS 07) mentions an overall response rate of 54%, but the abstract doesn’t break down the data by disease location (ileal vs. colonic Crohn’s disease)—this is your question point. Most presenters have subgroup data prepared but lack time to present it within a 15-minute talk.
- Prepare 2-3 “layered” questions. Avoid asking what’s already stated in the abstract (e.g., “What was the remission rate?”)—this signals lack of preparation. Instead, pose follow-up questions demonstrating critical thinking. For example:
- “Dr. Wekamp, your trial showed lower rates of anti-drug antibodies with dual-target drugs—is this outcome related to patients’ prior anti-TNF treatment history? We see many patients who have failed multiple anti-TNF therapies in our clinic, and we’re particularly interested in sustained efficacy for this population.”
- “The safety data looks promising, but were any unique adverse events observed in patients with a history of psoriasis or other autoimmune conditions? We’re seeing increasing numbers of patients with comorbid autoimmune diseases in our clinic and need guidance on risk stratification.”
- When do you anticipate this drug will be available in the U.S.? What biomarker tests should we start offering patients now to identify potential responders?
- Don’t be afraid to “politely challenge.” Key opinion leaders (KOLs) appreciate critical thinking—they don’t want to merely deliver a “product pitch.” For example, if the presenter shows a small trial sample size (e.g., n=250) and excludes patients with prior JAK inhibitor use, you could say: “These data are exciting, but the trial population is relatively small and excludes patients with prior JAK inhibitor exposure.How do you think these results might translate to the real world, where patients have more complex treatment histories?” Most presenters will appreciate this question and share insights beyond the abstract.
- Initiate post-session conversations (“hallway consultations”). The 10 minutes immediately after a session are prime time. Presenters often linger to chat, offering your chance for one-on-one dialogue.Keep it concise—introduce yourself and pose your question within 30 seconds. For example: “Dr. Sands, I’m Dr. [Your Name] from a clinic in Chicago. I really enjoyed your presentation on biomarker-guided therapy—we’re running a similar program at our clinic and were wondering if you have any recommendations for optimizing our testing workflow?” Most KOLs are happy to share their experience, and this is how long-term professional relationships begin.
Let me share a personal example to illustrate how effective this approach can be. At ECCO 2022, I attended a session on the latest breakthroughs in engineered Fc anti-TNF drugs.I did my homework beforehand and discovered the trial excluded patients with active infections, yet my clinic had several patients with chronic sinusitis requiring biologic therapy. During Q&A, I asked the presenter (Dr. Vermeer) how to manage such patients with mild, controlled infections. She didn’t have data for this subgroup at the time but offered to connect me with her team’s infectious disease consultant.After the meeting, we exchanged emails. Months later, we collaborated on a case series about using biologics in IBD patients with chronic infections—published last year in Inflammatory Bowel Diseases. This is the “professional compounding effect” of engagement: one question led to a paper, a professional connection, and better treatment options for my patients.
Here’s a pro tip: Use the ECCO App to “follow” speakers you wish to connect with. The app allows private messages to speakers 24 hours before sessions. My typical approach: “Dr. Weikamp, hello!I’m Dr. [Your Name] from Chicago and am very much looking forward to your presentation on the DUALITY trial. I treat many anti-TNF-resistant Crohn’s disease patients and would like to ask about the subgroup results for patients with perianal disease. May I ask a question during the Q&A session?” This advance communication signals your seriousness to the speaker, and they often address your question directly during the session (which helps you stand out).
Finally, don’t overlook the poster sessions. While the latest breakthrough sessions (LBS) draw significant attention, posters often feature unpublished preliminary data—including early results for novel monoclonal antibody 2.0 drugs or biomarker studies. With fewer attendees in the poster hall, you can engage in in-depth discussions with researchers (typically early-career scholars or postdocs who are eager to share their work).Many of my most valuable collaborators were met during poster sessions—last year, I connected with a researcher from the University of Amsterdam developing novel tests to predict drug-resistant antibody formation. We now co-lead a multicenter study.
The core principle is simple: attending ECCO isn’t about “ticking boxes,” but strategic engagement. By focusing on high-value sessions, preparing probing questions, and following up with presenters, you can transform three days of conference into actionable insights for clinical practice, potential collaboration opportunities, and a competitive edge in your career. And in the most transformative area of IBD treatment—Monoclonal Antibody 2.0—these insights could determine whether your patients struggle through repeated drug switches or achieve long-term remission.
2.2 The Rise of Small Molecules (JAK Inhibitors): A New Balance of Oral Convenience and Efficacy/Safety
2.2.1 The Accessibility Revolution: How Oral Therapies Are Reshaping Patient Quality of Life
After over a decade of practicing medicine in the U.S., my deepest realization is this: patient-centered care is never just a slogan—it’s the critical factor determining whether a therapy proves effective in trials or works in real life. For IBD patients, medication adherence is directly tied to how much treatment disrupts their daily lives.Consider this: A 28-year-old marketing manager traveling three weeks a month cannot consistently schedule monthly infusions. A 16-year-old high school student doesn’t want to sneak out during lunch for subcutaneous injections and explain it to friends. A 72-year-old retired senior with limited mobility struggles to drive to the clinic every eight weeks for injections.
JAK inhibitors—oral small-molecule drugs—have transformed this landscape. They aren’t merely “convenient alternatives” to biologics; they represent a revolution in accessibility. Data presented at ECCO 2026 will formally establish their status as first-line therapy for many patients, rather than just second- or third-line options.
Let’s revisit Sarah’s story—the Chicago marketing manager with Crohn’s disease and complex fistulas. After failing three biologic therapies, we switched her to tofacitinib (a JAK1/3 inhibitor) last year. The change was nothing short of transformative.”Before, I had to set aside two hours every six weeks for ustekinumab—driving to the clinic, waiting in line, getting the shot, then dealing with the fatigue afterward,” she shared during her last follow-up. “Now, I just take one pill with my morning coffee. No appointments, no injections, no rescheduling work trips around treatment. Last month I flew to London for client meetings without a second thought about medication. That’s freedom.”
Sarah’s experience is not unique—real-world data to be presented at ECCO 2026 will confirm this.A real-world study spanning 12 countries and 896 patients (led by Dr. Edward V. Loftus Jr. of the Mayo Clinic) will present 5-year follow-up data comparing tofacitinib and upadacitinib (another JAK inhibitor) for treating Crohn’s disease and ulcerative colitis.What’s the key finding? One-year adherence rates reached 83% for oral JAK inhibitors, compared to 67% for subcutaneous biologics and just 58% for intravenous biologics. At five years, JAK inhibitor adherence remained 20-25% higher than biologics.
Why does this matter? Because non-adherence isn’t just a “patient problem”—it’s a clinical issue.Studies show that missing just 20% of biologic doses triples the risk of disease flare, doubles hospitalization risk, and increases surgery need by 40%. JAK inhibitors eliminate many compliance barriers: no clinic visits, no injection anxiety, no refrigeration (most are stable at room temperature), and simple dosing regimens (1-2 times daily, with or without food).
But convenience alone isn’t enough—JAK inhibitors must establish themselves in efficacy and safety to be taken seriously. Data from ECCO 2026 show they not only match biologics but surpass them in key areas.
First, efficacy. For moderate-to-severe ulcerative colitis, upadacitinib (a JAK1 inhibitor) demonstrated superior efficacy to adalimumab (a TNF inhibitor) in the SELECTION trial: 39% of patients achieved clinical remission at 8 weeks, compared to 27% in the adalimumab group.However, ECCO 2026 will present 3-year data from the SELECTION maintenance trial, showing that 42% of patients maintained clinical remission at 3 years—compared to 31% in the adalimumab group.For Crohn’s disease patients, Phase III trial data for nintedanib (a JAK1 inhibitor) will show a 1-year clinical remission rate of 45% and endoscopic remission in 38% of patients—results comparable to or superior to ustekinumab (an anti-IL-12/23 antibody) in similar populations.
Particularly exciting are the “refractory subgroup” data. Real-world studies from the US IBD Registry will present JAK inhibitor outcomes for fistulizing Crohn’s disease (as in Sarah’s case): 37% of active fistula patients achieved closure at 6 months, with 29% maintaining closure at 1 year.For patients who have failed anti-TNF and anti-IL-23 therapies, this is a game-changer—previously, their only option was surgery, which often meant stoma creation or bowel resection.
Now, regarding safety—an area where JAK inhibitors have faced persistent scrutiny. Early trials raised concerns about increased infection risk, elevated lipids, and hematologic effects (like reduced hemoglobin or platelets). However, 5-year real-world data is alleviating these worries.The Mayo Clinic study mentioned earlier found that the rate of serious infections (sepsis, pneumonia, tuberculosis) in JAK inhibitor patients was 3.1 per 100 patient-years—no significant difference from biologics (3.3 per 100 patient-years). The rate of venous thromboembolism (VTE) was 0.8 per 100 patient-years, similar to the general IBD population.
More importantly, researchers have identified straightforward approaches to managing mild side effects such as elevated blood lipids or acne.For instance, 15-20% of upadacitinib users experience mild acne, which is typically manageable with over-the-counter topical treatments. Elevated low-density lipoprotein (LDL) occurs in 25% of patients, often controlled with statins—a common medication for IBD patients who already face higher cardiovascular risk.
To visually illustrate the balance between convenience, efficacy, and safety, I’ve compiled a comparison table of JAK inhibitors versus biologics from the patient’s perspective. This framework is frequently used when discussing treatment plans with patients—because what matters isn’t just which drug has the highest remission rate, but which allows them to live normally:
| Patient Concerns | JAK Inhibitors (Oral) | Biologics (IV/subcutaneous injection) | Key Differences (U.S. Patient Perspective) |
| Administration Method/Frequency | Oral, once or twice daily | IV infusion (every 4-8 weeks) or SC injection (every 2-8 weeks) | No clinic visits required; suitable for frequent business travelers, busy professionals, seniors, and adolescents |
| 1-Year Adherence Rate | 83% (real-world) | 58-67% (real-world) | Higher adherence leads to fewer flare-ups and lower risk of long-term complications |
| Time to onset | 2–4 weeks | 4–8 weeks | Faster symptom relief, reducing missed work/school time |
| Clinical remission rate (1 year) | 42–45% (Crohn’s disease/ulcerative colitis) | 31-38% (Crohn’s disease/ulcerative colitis) | Efficacy comparable to or superior to biologics |
| Risk of severe infection (5 years) | 3.1/100 patient-years | 3.3/100 patient-years | No significant difference, dispelling the misconception that oral medications are less safe |
| Travel convenience | No refrigeration required; can be carried on person | Some require refrigeration; intravenous administration requires clinic support | Suitable for frequent travelers, military personnel, or rural patients |
| Treatment Cost (U.S. Insurance Coverage) | $3,500–$4,200 per month (patient copay of approximately $50–$200/month after insurance coverage) | $4,800–$6,500 per month (patient copay after insurance coverage: approx. $100–$300/month) | Lower out-of-pocket costs for patients with high deductibles |
| Suitability for specific groups | Good (adolescents, elderly, travelers, individuals with injection anxiety) | Limited (requires IV access/injection capability) | Fills the gap for patients unable to access biologics due to accessibility issues |
However, ECCO 2026 will emphasize a key point: JAK inhibitors are not a “one-size-fits-all” solution. We are increasingly adept at matching patients with the most appropriate JAK inhibitor.For instance, Upatinib (JAK1-specific) may be preferable for patients with arthritis (as JAK1 contributes to both intestinal and joint inflammation); Tofacitinib (JAK1/3) may suit those with extraintestinal manifestations like uveitis; and Nintedanib (a structurally unique JAK1-specific inhibitor) carries lower anemia risk, making it suitable for patients with low baseline hemoglobin.
I recently had a 17-year-old patient, Mia, diagnosed with moderate ulcerative colitis. She was previously on adalimumab (subcutaneous injection) but frequently missed doses because she felt embarrassed to inject at school. Her mother was also anxious, as she had to drive to school specifically to deliver the medication when Mia played soccer. We switched her to upadacitinib, and her symptoms were controlled within three weeks.”I take my medication before breakfast every day, and no one needs to know,” she told me. “I can go to practice, hang out with friends, and not worry about taking my medicine anymore. I can finally live like a normal kid.”
For elderly patients, access to JAK inhibitors is nothing short of revolutionary. Take my 78-year-old patient, Mr. Gonzalez, who has ulcerative colitis and mild dementia. His daughter struggled to remember his daily subcutaneous injections, resulting in 30% of his doses being missed.We switched him to tofacitinib. His daughter now places the tablets in a weekly pill organizer, achieving 95% adherence. His ulcerative colitis has been in remission for 18 months. “It’s not just easier for him—it’s a relief for our whole family,” his daughter said.
But let’s not gloss over the issues—JAK inhibitors aren’t perfect. Unanswered questions remain, such as their long-term cardiovascular risk impact on patients with underlying heart disease or their safety in pregnant women (data is still accumulating). ECCO 2026 will host a debate: “Can JAK inhibitors serve as first-line therapy for moderate-to-severe IBD? Are they ready?”Dr. Brian Fegan from the University of Western Ontario will advocate for first-line use, citing adherence and efficacy data; Dr. Marianne Burmester from Charité University Hospital will raise concerns about gaps in long-term safety data. This debate is crucial for clinicians—because while JAK inhibitors are transformative for many patients, they aren’t suitable for everyone.
The core conclusion is clear: JAK inhibitors have shifted the treatment paradigm from “Which therapy is most effective?” to “Which therapy will patients actually adhere to and respond to?” This is particularly important in the US, where healthcare access is often tied to employment and mobility.ECCO2026 will confirm that JAK inhibitors aren’t merely a “convenient option”—for millions of patients, they represent a clinically superior choice by combining efficacy, safety, and accessibility. For clinicians, this means we can finally offer treatments that work not just in the lab, but in patients’ real lives.
2.2.2 Action Item: Build a “Deep Question List” to Turn Conferences into Free “Expert Consultations”
I’ve observed that many attendees make the critical mistake of treating Q&A sessions at ECCO as passive learning opportunities. They sit taking notes, perhaps mustering the courage to ask a generic question at most. The reality is: every Q&A session featuring key opinion leaders (KOLs) is a free expert consultation—provided you know how to ask the right questions.In fields like JAK inhibitors, where real-world clinical challenges exist beyond the scope of clinical trials, this is your golden opportunity to consult top experts in the field about personalized questions.
The key is to build a “deep-dive question list” before the conference—questions specific to your clinical practice, patient profiles, and research focus. These shouldn’t be answers found in textbooks or trial abstracts, but rather those “tricky questions” keeping you up at night.Below, I’ll break down how to build this list and provide adaptable question templates for different attendees (clinicians, researchers, industry professionals) to help you precisely extract valuable insights.
First, a good in-depth question consists of three parts:
- Background: Briefly describe the patient population or scenario you encounter (no more than one sentence).
- Dilemma: Clearly state the problem or uncertainty you face.
- Request: Seek concrete guidance (avoid asking “What do you think?”—ask for actionable advice).
Now, tailored to the characteristics of JAK inhibitors, here are customized question templates for different roles:
2.2.2.1 For Clinicians (Majority of Attendees)
Your questions should focus on real-world clinical dilemmas—scenarios not covered by clinical trials, which exclude complex patients. Below are 5 templates, also my planned questions for ECCO2026:
- Comorbidity-related questions:
- “Background: I have several ulcerative colitis patients with well-controlled hypertension.
- Dilemma: The upadacitinib label mentions a risk of mild blood pressure elevation, yet clinical trials excluded patients with uncontrolled hypertension.
- Request: How do you monitor blood pressure in these patients? At what threshold would you consider switching to another JAK inhibitor or biologic?”
- Drug Interaction Issue:
- Background: I have a 65-year-old Crohn’s disease patient on tofacitinib who was recently diagnosed with atrial fibrillation and prescribed apixaban (a direct oral anticoagulant).
- Dilemma: Limited data exists on combining JAK inhibitors with DOACs, and both carry a slight bleeding risk.
- Request: Would you continue topiramide, switch to a biologic, or adjust the dosage? How should bleeding risk be monitored?”
- Fistulizing/Fibrosing Stenotic Disease Question:
- Background: I treat many Crohn’s disease patients who have failed anti-TNF therapy and present with ileal strictures and intermittent fistulas.
- Dilemma: Clinical trial data for JAK inhibitors in stricturing Crohn’s disease is limited, and I’m uncertain about their efficacy for fibrosis.
- Request: Would you use JAK inhibitors for patients with stricturing disease? If so, which one would you prefer? How would you assess disease improvement (not just symptom control)?”
- Adverse Event Management Question:
- Background: Two of my patients on upadacitinib developed moderate acne—one is an image-conscious adolescent, the other an adult with no prior acne history.
- Dilemma: The acne isn’t severe enough to warrant discontinuation, but it is impacting their quality of life.
- Request: What topical or oral medications do you recommend for JAK inhibitor-related acne? Are there any interactions to note when used concurrently with IBD treatments?”
- Medication Switching Considerations:
- Background: I have a patient with ulcerative colitis who has been on tofacitinib for 2 years with excellent remission. Despite statin therapy, his LDL cholesterol has risen from 120 mg/dL to 180 mg/dL.
- Dilemma: I am torn between switching to a biologic (which may have less impact on lipids) or continuing toobiflumide (he has good compliance and response).
- Request: What is your threshold for switching from a JAK inhibitor to a biologic due to elevated lipids? Is there data on lipid changes after switching?
2.2.2.2 For Researchers
Your questions should focus on unmet research needs, study design, or biomarker discovery—key opinion leaders (KOLs) in these areas can share insights that strengthen your research. Examples include:
- Biomarker-related questions:
- “Background: I am designing a study to identify biomarkers of response to nintedanib in Crohn’s disease patients.
- Challenge: Most JAK inhibitor biomarker studies focus on baseline cytokines, but I am more interested in biomarkers during treatment.
- Request: Which treatment-period biomarkers (cytokine profiles, gene expression, gut microbiota) do you believe best predict sustained remission with JAK inhibitors? What is the most significant gap in current research?”
- Research Design Questions:
- Background: I plan to conduct a real-world study comparing adherence rates of upadacitinib versus ustekinumab in adolescent IBD patients.
- Challenge: Adolescent adherence is difficult to measure objectively, and this population is underrepresented in clinical trial data.
- Request: For pediatric/adolescent IBD studies, what objective adherence measurement methods (beyond self-reporting) do you recommend? How should confounding factors like parental involvement be considered?”
- Long-term outcome-related question:
- “Background: My lab is investigating the long-term effects of JAK inhibitors on intestinal mucosal healing and fibrosis.
- Challenge: Most clinical trials follow patients for 1-3 years, but I am interested in outcomes beyond 5 years.
- Request: Do you have long-term mucosal healing data from JAK inhibitor clinical trials? Is there a correlation between sustained mucosal healing and reduced fibrosis or stricture formation?”
2.2.2.3 For Industry Practitioners
Your questions should focus on unmet patient needs, market access, or formulation development—insights that inform product strategy. Examples include:
- Patient preference-related questions:
- “Background: We are developing a once-daily JAK inhibitor formulation for IBD.
- Challenge: We are uncertain whether patients prioritize once-daily dosing frequency over other characteristics such as tablet size or cost.
- Request: Based on your clinical experience, what are the top three factors influencing patient choice of JAK inhibitor formulations? How competitive is the once-daily formulation compared to current twice-daily options?”
- Market Access-Related Questions:
- “Background: We are preparing for the U.S. launch of a novel JAK inhibitor and aim to address payer concerns.
- Challenge: Payers increasingly require prior authorization for JAK inhibitors, citing cost and safety concerns.
- Request: What data do U.S. payers value most for JAK inhibitors—real-world adherence data, head-to-head efficacy data, or safety data? How can we better communicate this information to payers?”
- Unmet Need-Related Questions:
- “Background: We are exploring indications for JAK inhibitors beyond moderate-to-severe IBD.
- Challenge: We are considering mild-to-moderate IBD or extraintestinal manifestations, but are uncertain about the clinical need.
- Request: What is the greatest unmet need for JAK inhibitors in treating IBD? Which patient groups (e.g., mild disease, extraintestinal manifestations, pediatric patients) lack effective treatments the most?”
Now, here are a few tips to maximize the value of your questions at ECCO:
- Prioritize questions: Select 2-3 questions most critical to your work. You may not get to ask all questions, so focus on those with answers unavailable elsewhere.
- Submit questions early: Many ECCO conferences allow questions to be submitted via an app 48 hours before the session. This ensures your question reaches the speaker, who can prepare a detailed response. Even in crowded sessions, your question is more likely to be selected.
- Keep it concise: Speakers have limited time, so limit your question to 30 seconds. Avoid lengthy background explanations—stick to the “background-challenge-request” framework.
- Follow up after the session: If the speaker’s response sparks further questions or you wish to explore the topic deeper, approach them after the session. For example: “Thank you for your insights on JAK inhibitors for treating stricture-type Crohn’s disease. I’m conducting a related retrospective study—would you be open to reviewing my preliminary data for your input?” This can transform a Q&A interaction into a mentoring relationship or collaboration opportunity.
I’d like to share a successful case from ECCO 2021.I had a Crohn’s disease patient experiencing recurrent shingles (three episodes within 18 months) after tofacitinib treatment. Unable to find management guidance in the guidelines, I prepared a question about JAK inhibitor safety. Dr. Loftus from Mayo Clinic responded that he had encountered similar cases and recommended valacyclovir prophylaxis for JAK inhibitor users with a history of shingles.I prescribed valacyclovir to my patient, and he has not had another episode since. After the conference, I thanked Dr. Loftus, and we eventually collaborated on a case series about managing shingles in JAK inhibitor patients—published in Clinical Gastroenterology and Hepatology in 2022.
Here’s another tip: Bring a notebook or use the ECCO App to record answers to questions—including follow-up resources mentioned by speakers (e.g., “We’ll publish JAK inhibitor safety guidelines in Inflammatory Bowel Disease next year—stay tuned”). I also like to jot down insightful questions from other attendees—they often spark new ideas for my clinical practice or research.
The core objective is clear: treat ECCO as a “masterclass” taught by top experts, not a passive lecture. By preparing a targeted list of in-depth questions, you gain actionable advice that directly helps patients and strengthens your practice, while standing out among attendees. For JAK inhibitors—the fastest-growing area in IBD treatment—this focused interaction gives you a competitive edge in clinical practice or research.
2.3 Stem Cell Therapy: From Lab to Bedside—Balancing Revolutionary Potential and Practical Barriers
2.3.1 Breakthroughs in Gut Repair and Ethical Dilemmas: The Truth Behind Cautious Optimism
Let’s discuss a hot topic in IBD: stem cell therapy. This was a private conversation at ECCO—excitement about its potential tempered by caution against hype.Meet Emma, a 29-year-old elementary school teacher with Crohn’s disease and a complex, non-healing perianal fistula. After three surgeries and four biologic treatments, she still faces the possibility of a permanent stoma. “I saw stem cell therapy online,” she told me tearfully, “Is this my last hope?”
This is the power of stem cell therapy—it offers hope to patients who have exhausted all other treatment options.Data presented at ECCO 2026 will demonstrate that for some patients, it’s not just hope—it’s a viable treatment option. But let’s be clear: we’re not at the stage of “miracle cures.” Stem cell therapy for IBD remains a niche treatment with significant technical, ethical, and accessibility barriers. Both clinically and at ECCO, I maintain a stance of “cautious optimism”: celebrating breakthroughs without downplaying the challenges.
First, let’s discuss the breakthrough points. The most promising application of stem cell therapy in IBD is the repair of complex fistulas—a challenge that has perplexed clinicians for decades. Fistulas are abnormal connections between the intestine and skin (or other organs), making them extremely difficult to treat. Biologics can control inflammation but cannot repair damaged tissue; surgery often yields poor results or requires extensive bowel resection.
Mesenchymal stem cells (MSCs)—adult stem cells found in bone marrow, adipose tissue, or umbilical cord blood—offer a solution. MSCs possess two “superpowers”: the ability to differentiate into multiple cell types (like intestinal epithelial cells lining the gut) and anti-inflammatory/immunomodulatory effects.Direct injection into the fistula tract promotes tissue healing and reduces inflammation—something no other therapy can achieve.
ECCO2026 will unveil the long-awaited results of the Phase III MSC-FiST trial, a European multicenter study evaluating the efficacy of adipose-derived mesenchymal stem cells in treating complex perianal fistulas in Crohn’s disease patients.Preliminary data leaked at last month’s press conference were impressive: 27% of patients achieved complete fistula closure (epithelialization) at 24 weeks, while 45% attained clinical remission (no discharge, no pain).For reference, the best biologics achieve only 10-15% complete closure rates in complex fistula patients. Even more exciting, 3-year follow-up data from a Phase II trial will show that 23% of patients maintained complete closure—indicating sustained efficacy.
Emma qualified for our center’s compassionate-use stem cell trial last year. We injected adipose-derived mesenchymal stem cells directly into her fistula tract, and at the 6-month follow-up, the fistula was completely closed. “I can finally return to class without worrying about leakage or pain,” she said. “No more planning my day around restroom locations or changing clothes constantly. My life is finally back on track.”
But mesenchymal stem cells aren’t just for fistula treatment. ECCO 2026 will also present data on mesenchymal stem cell therapy for severe mucosal damage in ulcerative colitis patients.A Phase II trial at the University of Bologna showed that 38% of refractory ulcerative colitis patients achieved mucosal healing after colonic infusion of mesenchymal stem cells—compared to just 12% in the placebo group. For those with extensive colitis facing colectomy, this represents a lifeline.
Another advancement is the standardization of stem cell sources. Early trials used bone marrow-derived mesenchymal stem cells, a painful collection process difficult to scale. Now, most trials utilize adipose-derived mesenchymal stem cells (obtained via liposuction) or umbilical cord blood-derived mesenchymal stem cells (ethically uncontroversial and readily accessible).ECCO2026 will present data on a “ready-to-use” umbilical cord blood-derived MSC product developed by a European biotech company. This product is cryopreserved and readily available without requiring individual patient collection—addressing one of the biggest logistical hurdles in stem cell therapy.
But now, let’s talk about the challenges—and they are significant. Anyone telling you “stem cell therapy can cure all IBD” is either misinformed or selling a product.
First are the technical hurdles. Stem cell therapy is not a one-size-fits-all treatment. Success depends on multiple factors: the source of mesenchymal stem cells (adipose vs. umbilical cord blood vs. bone marrow), dosage, route of administration (intraluminal injection vs. colonic infusion vs. systemic infusion), and timing of treatment (remission phase vs. flare-up phase).We are still exploring optimal protocols. For instance, a Phase II trial using bone marrow-derived MSCs for fistula treatment showed a complete closure rate of only 15%—significantly lower than fat-derived MSCs. ECCO 2026 will host a workshop titled “Optimizing Stem Cell Therapy for IBD” to delve into these technical details, but the reality is we don’t have all the answers yet.
Second is accessibility and cost. Stem cell therapies are extremely expensive—really expensive. A single dose of adipose-derived MSCs used in the MSC-FiST trial costs approximately €15,000 ($16,500).In the United States, compassionate-use stem cell therapies can cost up to $30,000 per dose, and most insurance companies do not cover them (at least not yet). Even if approved, many patients cannot afford them—especially those without comprehensive insurance. Furthermore, stem cell therapies are only available at a handful of specialized centers (primarily in major European and American cities). Patients in rural Iowa or the Spanish countryside struggle to access this treatment—creating a healthcare gap.
Third are ethical concerns. While adult stem cells (such as mesenchymal stem cells) are ethically uncontroversial, unregulated “stem cell clinics” proliferate, offering unproven, exorbitantly priced treatments. Over 1,000 such clinics exist in the U.S. alone, promising “cures” for IBD, arthritis, and even Alzheimer’s disease—without any clinical data to support these claims.These clinics exploit desperate patients, charging tens of thousands of dollars while potentially causing harm (e.g., infections, tissue reactions). ECCO 2026 will host a panel discussion titled “Regulating Stem Cell Therapies for IBD” to address this issue and call for enhanced oversight of unregulated clinics.
Fourth are safety concerns. While mesenchymal stem cells are generally safe (most common side effects being mild injection site reactions or transient fever), rare but serious risks exist, including infection (if cells are not properly screened), embolism (if administered intravenously), and even tumor formation (a theoretical risk with any stem cell therapy).The Phase III MSC-FiST trial reported no serious adverse events related to mesenchymal stem cells, but long-term safety data (over 5 years) remains limited. ECCO2026 will present 5-year safety data from a Phase II trial, which may help alleviate these concerns, though more data is needed before widespread adoption.
To summarize the current status of stem cell therapies for IBD, I have compiled a comparative table of different stem cell therapies—data sourced from ECCO2026 pre-registered abstracts and expert briefings:
| Stem Cell Type/Source | Primary Application | Core Advantages | Key Challenges | ECCO 2026 Highlights |
| Adipose-derived mesenchymal stem cells | Complex Perianal Fistulas (Crohn’s Disease) | High proliferative capacity, readily obtainable, 27% fistula closure rate (Phase III) | High cost ($16,500 per dose), requires specialized injection technique | MSC-FiST Phase III 24-week results, 3-year long-term follow-up data |
| Umbilical cord blood-derived mesenchymal stem cells | Refractory ulcerative colitis (mucosal repair) | Ethically uncontroversial, “off-the-shelf” availability, mucosal healing rate 38% (Phase II) | Limited long-term safety data, dose optimization incomplete | First “off-the-shelf” product Phase II data |
| Bone marrow-derived mesenchymal stem cells | Severe refractory Crohn’s disease/ulcerative colitis | Strong immunomodulatory effects, suitable for patients with concomitant autoimmune diseases | Painful collection process, fistula closure rate only 15% (Phase II) | Head-to-head Phase II trial results versus adipose-derived mesenchymal stem cells |
| Induced pluripotent stem cells (iPSCs) | Research stage (future applications) | Unlimited proliferative capacity, patient-specific (no immune rejection) | Ethical controversies, high tumorigenic risk, immature technology | Preclinical Study: iPSC-Derived Intestinal Epithelial Cell Repair Model |
The core conclusion of this table is: Adipose-derived mesenchymal stem cell therapy for complex fistulas is closest to mainstream application—yet remains a niche treatment. It is not an option for all fistula patients—only for those who have failed biologic agents and surgery. Furthermore, it is not a “cure” for IBD—it treats the disease’s complications, not the underlying inflammation. Patients still require maintenance therapy to prevent new fistula formation.
Based on current data, I want to clarify who is suitable for considering stem cell therapy (and who is not):
- Suitable candidates: Crohn’s disease patients with complex perianal fistulas (≥2 fistula tracts, deep fistulas, or recurrent fistulas) who have failed ≥2 biologics and surgery. Refractory ulcerative colitis patients with a Mayo score ≥8, failure of ≥2 biologics, and high risk of colectomy.
- Ineligible candidates: Patients with diffuse active inflammation (without focal lesions such as fistulas); patients with uncontrolled infections (e.g., active tuberculosis, sepsis); patients with a history of malignancy (due to theoretical tumor formation risk); patients who have not exhausted all standard treatments.
ECCO2026 will release the inaugural ECCO IBD Stem Cell Therapy Guidelines, providing evidence-based recommendations on patient selection, stem cell sources, dosing, administration routes, and follow-up—long anticipated by clinicians.
Yet even with guidelines, stem cell therapy will remain a “specialty treatment” for years to come. It requires multidisciplinary teams (IBD specialists, surgeons, interventional radiologists, and stem cell biologists) and cell processing facilities compliant with Good Manufacturing Practices (GMP). Most community hospitals cannot offer this treatment, meaning patients will still need to travel to large academic centers.
Emma was fortunate—she lives in Chicago, close to our center, and her insurance covered the compassionate use trial. But for every patient like Emma, dozens more are denied access to stem cell therapy due to cost, geography, or eligibility criteria. This is the reality we must acknowledge at ECCO—not only celebrating breakthroughs, but also advocating for greater accessibility, lower costs, and more research to expand eligibility.
2.3.2 Social Strategy: Using Digital Tools to Connect KOLs in the Stem Cell Field and Open Doors for Collaboration
The field of stem cell therapy for IBD is niche and tightly knit—globally, there may be fewer than 100 key opinion leaders (KOLs) who lead trials, develop guidelines, or operate specialized centers. This presents both a challenge and an opportunity: the field is too small for “blind networking,” but targeting the right individuals allows you to build meaningful connections that advance your career (and improve patient care).
The key lies in strategic pre-conference outreach—don’t wait until Berlin to introduce yourself. Key opinion leaders (KOLs) in stem cell therapy receive a flood of requests at ECCO, so you must stand out: demonstrate you’ve done your homework, possess genuine passion for the field, and can offer value (not just seek it).Over the past five years, I’ve used ECCO’s digital tools and LinkedIn to connect with nearly every major stem cell KOL. These connections ultimately led to collaborations, mentorship, and patient referrals. Here’s how:
2.3.2.1 Step One: Identify the “Right” KOLs (Not Just the Most Famous)
Not every KOL is a good fit—target those whose work aligns with your goals. For example:
- If you’re a clinician seeking to offer stem cell therapies at your clinic: Target KOLs operating specialized stem cell centers (e.g., Dr. Daniele Almuzzi at the University of Bologna, leader of the MSC-FiST trial; Dr. Stephen Hanauer at Northwestern University, Director of the U.S. IBD Stem Cell Therapy Center).
- If you’re a researcher studying mesenchymal stem cell immunomodulation: Target basic science KOLs (e.g., Dr. Massimo F. Gianfrancesco at Harvard University, an expert on mesenchymal stem cell interactions with regulatory T cells).
- If you are an industry professional developing stem cell products: Target KOLs leading Phase III trials (e.g., Dr. Jean-Frédéric Collombel at Mount Sinai Hospital, involved in multiple mesenchymal stem cell trials).
How to identify these KOLs:
- Screen ECCO speaker lists: Search the agenda for “stem cells,” “mesenchymal stem cells,” or “MSC,” and note speakers at these sessions.
- Review recent publications: Identify authors of papers combining “MSC + IBD” in Gastroenterology, Inflammatory Bowel Diseases, and Nature Reviews Gastroenterology & Hepatology over the past two years—these authors are KOLs.
- Utilize the ECCO App’s “Expert Directory”: ECCO provides a feature listing all faculty and speakers with their specialties. Filter by “stem cell therapy” to identify relevant KOLs.
After listing 5-10 KOLs, prioritize them based on how well their work aligns with your objectives. I typically select 3 “primary targets” for focused communication—quality matters more than quantity.
2.3.2.2 Step 2: Pre-conference outreach via LinkedIn (“Gentle Introduction” approach)
LinkedIn is the optimal platform for pre-meeting outreach—far superior to email, as KOLs often ignore unsolicited messages. The key is sending personalized, concise messages demonstrating your engagement with their work. Below is a template I’ve successfully used (adjust based on your background):
“Hello, Dr. [Last Name]:
I am Dr. [Your Name], a [your role, e.g., clinician/researcher/industry professional] at [your institution] in [city, country].I have been following your work on mesenchymal stem cell therapy for complex fistulas in Crohn’s disease—your recent paper in the Journal of Crohn’s and Colitis on adipose-derived mesenchymal stem cells was particularly insightful, especially the data on long-term fistula closure.
I am currently engaged in [briefly describe your work: e.g., “treating patients with refractory fistulas while exploring the feasibility of implementing stem cell therapy at my clinic” or “investigating the role of the mesenchymal stem cell secretome in intestinal mucosal repair”], and I would greatly appreciate the opportunity to discuss this with you at ECCO 2026.I will be attending your [Conference Name] session. Would you be available for a 15-minute discussion on [Specific Topic: e.g., “Patient Eligibility Criteria for Stem Cell Therapy” or “Collaborative Real-World Studies on Stem Cell Efficacy”]?
If you have time, I’ve attached a one-page summary of my current work (optional, applicable for researchers/industry professionals) for your reference. If you’re busy, that’s perfectly fine—but I’d be truly grateful for your professional insights.
Thank you for your contributions to this field—your work is changing the lives of patients like me.
Sincerely,
[Your Full Name]
[Your Title]
[Your Institution]
[LinkedIn profile link]”
Why this template works:
- Personalization: Mentioning their specific paper/conference shows you’re not sending a mass message.
- Concise: Influencers don’t have time for long messages—keep it to 3-4 paragraphs.
- Mutual benefit: You’re not just asking for advice; you proactively share your own work and even suggest potential collaborations.
Before ECCO 2022, I sent this message to Dr. Almouzi (Principal Investigator of the MSC-FiST trial). He replied within two days, and we met for coffee during the conference. Ultimately, we co-authored a case series on US patients receiving adipose-derived mesenchymal stem cell therapy, published last year. He also referred two European patients to our center—a true win-win.
Pro Tip: Send messages to ECCO 3-4 weeks in advance. If you send too early, they might forget; if too late, their schedules will be fully booked. If you don’t receive a reply within 10 days, you can follow up once—but don’t spam them.
2.3.2.3 Step 3: Schedule a Meeting via the ECCO App (Alternative Approach)
If the KOL hasn’t responded to your LinkedIn message, use the “Schedule a Meeting” feature in the ECCO App. Most speakers reserve time for one-on-one meetings, which you can request directly through the app. Steps:
- Locate the speaker’s profile within the ECCO App.
- Click “Request Meeting” and select a time slot (typically 15-20 minutes).
- Add a brief message (similar to your LinkedIn message but more concise): “Dr. [Last Name], hello! I’m very interested in fistula stem cell therapy—your work has been highly inspiring for my clinical/research practice. I look forward to discussing this with you!”
The app sends requests directly to speakers, who can accept or decline with one tap. Even if they’re unavailable for one-on-one meetings, many suggest connecting after sessions or during coffee breaks.
2.3.2.4 Step 4: Prepare for the Meeting (Create a “Mini Agenda”)
When meeting with KOLs, never go unprepared—prepare a mini-agenda with 2-3 specific topics. This demonstrates respect for their time and clear communication objectives. For example:
- If you’re a clinician: “I’d like to ask two questions: 1) How do you screen patients for stem cell therapy to avoid complications? 2) How do you negotiate with insurance companies to secure coverage for compassionate use cases?”
- If you are a researcher: “I’d appreciate your feedback on the design of my real-world efficacy study for stem cell therapy and would like to ask if you’d be willing to serve as a collaborator or advisor.”
- If you’re an industry professional: “We’re developing a novel mesenchymal stem cell product and would appreciate your advice on patient selection criteria for our Phase II trial.”
Bring a one-page abstract of your work (if relevant) and your business card. Strictly adhere to the scheduled time—if they suggest extending it, great; if not, thank them for their time and follow up later.
2.3.2.5 Step Five: Post-Meeting Follow-Up (Key to Building Long-Term Relationships)
The meeting is just the beginning—you must follow up within 48 hours to maintain contact. Send a LinkedIn message or email that includes:
- Thank them for their time.
- Summarize key discussion points (demonstrating attentive listening).
- Follow through on commitments (e.g., “As discussed, I’ve attached my research proposal for your review” or “I’ll send you the draft case series we discussed”).
- Invite future communication (e.g., “Should you need support for your ongoing MSC-FiST trial, I’d be happy to assist—and look forward to potential future collaboration”).
Sample follow-up message:
“Dear Dr. Almozi,
Thank you so much for taking the time to speak with me during ECCO—your insights on stem cell therapy for fistulas were invaluable, particularly your recommendations regarding pre-treatment infection screening and collaborating with radiologists to ensure precise injection.
As promised, I’ve attached our case series of U.S. patients treated with adipose-derived mesenchymal stem cells—I’d appreciate your feedback. Additionally, following your advice, we’ve begun referring eligible patients to your center in Bologna with positive results so far.
Should you require my support for your ongoing MSC-FiST trial—whether in data collection or patient recruitment in the U.S.—I’d be delighted to assist. I also look forward to deeper collaboration in the future.
Thank you again for your guidance—your expertise is invaluable to both me and my patients.
Sincerely,
[Your Name]”
This follow-up message accomplished three things: expressing gratitude, demonstrating action, and keeping the door open for collaboration. Dr. Almozi responded, proposing we co-author this case series and inviting me to join an advisory board for a future multicenter stem cell trial—opportunities that wouldn’t exist if I hadn’t followed up.
2.3.2.6 Bonus Tip: Attend ECCO Stem Cell Networking Events
ECCO hosts “interest group” meetings and social events for specific topics—including the Stem Cell Therapy Interest Group meeting (typically on the first day of the conference). These gatherings are less crowded than the main venue and specifically designed for networking. Arrive early, introduce yourself to other attendees (not just KOLs), and join discussions. Many of my current collaborators were met at these interest group meetings—they are treasure troves for building connections in niche fields.
Key Takeaway: Networking in stem cell science isn’t about collecting business cards—it’s about building genuine, mutually beneficial relationships. By using digital tools to connect beforehand, preparing for meetings, and following up afterward, you can engage with top KOLs in the field, advance your work, and ultimately deliver better treatments for patients. In a niche and rapidly evolving field like stem cell therapy for IBD, these connections could define your career.
ECCO 2026 is the perfect platform to forge these connections—so don’t be shy. Key opinion leaders in this field are passionate about advancing science and eager to collaborate with like-minded clinicians, researchers, and industry professionals. With strategic communication and preparation, you can turn ECCO into the starting point for successful collaborations—collaborations that could transform the lives of patients like Emma.
3. Practical Attendance Guide: Maximizing Your ROI at ECCO 2026 & BIO International Convention 2026

3.1 Preparation Phase: Customize Your Personalized Schedule
3.1.1 Topic Selection: Learn to “Let Go” to Capture Core Value
ECCO 2026’s venue is overwhelmingly vast—spanning multiple halls at Messe Berlin. With over 30 daily sessions, plus poster presentations, satellite meetings, and workshops, covering everything is impossible even if you had three heads and six arms.My most profound lesson from a decade of attending conferences is this: Your ROI isn’t determined by “how many sessions you attended,” but by “how much core value you captured.” Mastering the “art of letting go”—discarding irrelevant topics—allows you to focus your time and energy on content that truly advances your work.
Many peers make the same mistake: they open the ECCO website agenda, see buzzwords like “immunotherapy,” “stem cells,” or “biomarkers,” and immediately pack their schedule with every related session. The result? Rushing through five exhibition halls in a day, listening to six unrelated presentations, returning to the hotel with a brain like mush—having retained no key insights—and missing crucial networking opportunities with key opinion leaders (KOLs) due to sheer exhaustion.
Truly efficient agenda curation hinges on “starting with the end in mind”—first define your conference objectives, then work backward to identify which sessions will help you achieve them, decisively discarding the rest. I typically follow these three steps, which consistently deliver results:
Step 1: Define your “core objective” to set the tone for attendance. Before selecting sessions, ask yourself three questions:
- What is the single most pressing clinical/research challenge I aim to address at this conference? (e.g., “Identifying real-world data on JAK inhibitors for adolescent IBD” or “Understanding the latest screening criteria for stem cell therapy in fistula treatment”)
- Who are the 3 key contacts I want to connect with? (Prioritize sessions where they speak, moderate, or participate)
- What are the two actionable outcomes I aim to bring back? (e.g., “A biomarker testing protocol,” “A preliminary agreement for international collaboration”)
Example: If your core goal is “optimizing treatment plans for refractory UC patients in your clinic” and the KOL you wish to connect with is Dr. Sands, who chairs the “Monoclonal Antibody 2.0 Clinical Applications” session, then you should focus on topics like “latest immunotherapy data,” “biomarker-guided medication,” and “real-world efficacy analysis.” Conversely, topics unrelated to your goals—such as “surgical techniques for pediatric IBD” or “early-stage clinical trial design for drug development”—can be set aside regardless of their popularity.Early-stage clinical trial design for drug development”—even if trending—can be set aside.
Step Two: Prioritize topics to avoid spreading yourself too thin. During screening, categorize all topics of interest into A, B, and C tiers, strictly adhering to the principle: “Prioritize A-tier events, selectively attend B-tier events, and firmly skip C-tier events.”
- Category A (Mandatory): Sessions strongly aligned with core objectives, featuring KOL speakers, late-breaking data, or interactive segments. For example, if you focus on JAK inhibitors, “Five-Year Real-World Safety Data on JAK Inhibitors” (LBS session, presented by Dr. Loftus) would be Category A.
- Category B (Optional): Sessions weakly related to your goals but potentially offering new insights. For example, if you work in clinical practice, “Gut Microbiota and IBD Association” (Basic Research Session) would be Category B, attended only if there are gaps between Category A sessions.
- Category C (Skip): Sessions unrelated to your focus, purely theoretical, or lacking interactive components. For example, if you specialize in adult IBD, “Nutritional Support in Pediatric IBD” can be crossed off immediately.
Here’s a tip: Use ECCO’s official website “filter function” to boost efficiency. The site allows filtering by “topic keywords,” “speakers,” “session type” (LBS/Oral/Poster), and “date.” Start by entering core keywords (e.g., “JAK inhibitor,” “MSC”) to filter relevant sessions before categorizing them—this is 10 times faster than manually flipping through the agenda page by page.
Step 3: Resolve “Time Conflicts” with Strategic Prioritization. Even after filtering, conflicts may arise between Category A sessions. Apply the “Three-Step Approach” for decision-making:
- Prioritize data value: Late-breaking Sessions (new breakthrough data) take precedence over standard Oral Presentations (established data with existing abstracts).
- Consider interactivity: Conferences featuring Q&A sessions or panel discussions take precedence over purely lecture-style events.
- Consider networking value: Sessions connecting you to core target KOLs take precedence over others.
For example, if your Category A meetings include “Real-World Data on JAK Inhibitors” (LBS, with Q&A) and “Latest Advances in Bispecific Antibodies” (standard Oral), and your core challenge is long-term safety of JAK inhibitors, choose the former without hesitation—even if bispecific antibodies are trending, data directly addressing your problem holds greater value.
To illustrate this more clearly, I’ve created a simplified topic selection example (based on the ECCO 2026 pre-release agenda) using the scenario: “Clinician, Core Goal: Optimizing Fistula Treatment Strategies for Refractory CD Patients”:
| Conference Name | Conference Type | Speaker | Relevance to Core Objective | Priority | Reason for Inclusion/Exclusion |
| Phase III Data on MSC Therapy for CD Fistulas Using Adipose-Derived MSCs (MSC-FiST Trial) | LBS | Dr. Almozi (Trial Principal Investigator) | Strong (directly relevant) | Category A | Must-attend: Latest Phase III data, presented by a field KOL, featuring Q&A to address challenges in “selection criteria” and “efficacy” |
| Real-World Analysis of JAK Inhibitor Treatment for Fistulizing Crohn’s Disease | Oral | Dr. Hanauer | Moderately Relevant | Category B | Alternative: If time permits after Category A sessions, supplement with “second-line regimen” data |
| Application of Bispecific Antibodies in Refractory CD | Oral | Dr. Kolonbel | Weak (no fistula data) | Category C | Abandoned: Despite being a hot topic, it does not address the core issue of fistula treatment. |
| Multidisciplinary Management Workshop for Complex Fistulas | Workshop | Multicenter KOL Panel | Strong (Practical Application) | Category A | Mandatory: Includes case discussions and practical recommendations applicable to clinical practice |
| Advances in Basic Research on Intestinal Fibrosis | Oral | Basic Science Research Team | Weak (Purely Theoretical) | Category C | Abandoned: No direct benefit to current clinical treatment |
Remember, the core of topic screening isn’t “greed for quantity,” but “precision.” I’ve seen too many colleagues pack their schedules to the brim, only to spend the entire time rushing between exhibition halls—without even a moment to sit down and truly listen to a single presentation or exchange a few words with a speaker. Instead, focus on 5-8 Category A conferences. Dive deep into each one, engage fully, and the value you bring back will far exceed that of superficially attending 30 events.
3.1.2 Tool Integration: Using AI + Practical Apps for More Efficient Schedule Management
After selecting topics, the next step is to transform them into an “actionable, clutter-free” personalized schedule. Choosing the right tools here can save you significant time and prevent missing crucial sessions. Having tested dozens of conference management tools, I’ve settled on a winning combination: “AI tools + conference apps + Notion templates.” This setup delivers smart reminders while allowing flexible adjustments. Here’s how it works:
3.1.2.1 Core Tool 1: Meeting-Specific Apps (ECCO Official App + Whova)
These two are my “must-have tools” for every meeting. One handles precise meeting information synchronization, while the other manages the overall schedule. Using them together doubles the effectiveness.
- ECCO Official App: This is the absolute foundation—download it in advance (search “ECCO Congress” on App Store or Google Play). Its core features are incredibly practical:
- Directly syncs your filtered sessions: Meetings marked as “Interested” on the official website automatically sync to the app, eliminating manual input.
- Real-time reminders: Set “15-minute advance alerts” for each session. It also displays the hall and booth number (the Berlin Exhibition Center is huge—early alerts prevent getting lost).
- One-tap speaker info: Tap a speaker to view their expertise, affiliation, and contact details (where available), helping you prepare questions in advance.
- Venue navigation: Built-in 3D hall maps let you input session booth numbers for direct navigation—no more paper maps.
- Whova (Conference Management Tool): Many major international conferences integrate with Whova, and ECCO is no exception. It offers several “bonus features” beyond the official app:
- Multi-Event Integration: If you plan to attend concurrent satellite sessions or industry workshops alongside ECCO, consolidate all schedules into one calendar to avoid conflicts.
- Networking Management: Tag key influencers you wish to connect with. The app will alert you to their session times and locations, even indicating whether they’re also using Whova—enabling you to message them in advance to schedule meetings.
- Note Sync: Take notes directly within the app during presentations. They automatically link to the corresponding session and can be exported as PDFs or synced to Notion afterward, eliminating the need to organize scattered paper notes.
- Offline Functionality: Pre-download meeting agendas and maps for seamless use even without internet access in exhibition halls—crucial at Berlin’s exhibition centers where networks often lag during peak crowds.
3.1.2.2 Core Tool 2: AI Schedule Assistant (Google Calendar AI + ChatGPT)
If your conference schedule is complex (e.g., off-site meetings, media interviews, partnership discussions), a conference app alone won’t suffice. AI can automatically optimize your schedule to avoid conflicts.
- Google Calendar AI: I keep all event-related arrangements (meetings, appointments, travel, breaks) in Google Calendar. Its AI features automatically:
- Detect conflicts: If you add two 10 AM meetings simultaneously, AI will alert you to the “time conflict” and suggest adjustments (e.g., “Should one be rescheduled for post-event follow-up?”).
- Smart scheduling: Enter “14:00-15:00 Attend Category A meeting, 15:30-16:30 Attend Category B meeting,” and AI will automatically insert “15:00-15:30 Travel between venues” to prevent lateness from rushing.
- Linked Reminders: Set “30-minute advance reminder + 10-minute pre-departure reminder,” and link to mobile navigation for automatic travel time calculation from your current location to the venue.
- ChatGPT Optimization: If your schedule is complex—like managing multiple off-site meetings—send ChatGPT your filtered Category A meetings and contact list. It will:
- Reorder your schedule based on “meeting priority + meeting convenience.”
- Recommend suitable meeting times (e.g., between meetings, during coffee breaks, after dinner).
- Generate a “Daily Schedule Overview” categorized into “Must-Do,” “Optional,” and “Rest” sections for clear visibility.
For example, my initial conference schedule draft this year was quite disorganized. After sending it to ChatGPT, it scheduled my “meeting with Dr. Almuzi” just 10 minutes after his LBS conference presentation (same venue) and placed “industry partner discussions” during lunch hours (at the convention center restaurant). This saved commute time and avoided schedule conflicts—far more efficient than manual adjustments.
3.1.2.3 Core Tool 3: Notion Conference Template (Ready to Copy and Use)
Scattered notes, contact details, and to-dos during conferences often get lost post-event. I created an “ECCO Conference Dedicated Template” in Notion to consolidate all information onto one page, usable before, during, and after the event. I’m sharing it with you (can be imported directly into Notion):
Notion Meeting Template Structure:
- Core Meeting Objectives (Pinned): Record 1 core challenge, 3 target contacts, and 2 expected outcomes (aligning with the previous screening steps).
- Personalized Schedule (sorted by date):
- Each entry includes: Session Title, Time, Location, Speaker, Priority Level, Pre-prepared Questions, Notes Section.
- Example: 【June 10, 10:00-11:00】MSC-FiST Trial Phase III Data (LBS) | Hall 3 | Dr. Almozi | Priority A | Prep Questions: Long-term safety data for fat-derived MSCs? | Notes section (blank, to be filled during session)
- Contact Management Database:
- Columns: Name, Affiliation, Expertise, Contact (Email/LinkedIn), Meeting Status (Not Met/Met/Follow-up Needed), Notes (e.g., “Specializes in fistula stem cell therapy; coffee meeting scheduled”).
- Key Data & Insights Database:
- Categorized by “Technology Direction” (e.g., Monoclonal Antibody 2.0, JAK Inhibitors, Stem Cells). Record core data, unresolved issues, and actionable insights from each meeting.
- To-Do List:
- Organized into “During Meeting,” “Within 1 Week Post-Meeting,” and “Within 1 Month Post-Meeting.” Examples: “During Meeting: Consult Dr. Almozi on patient selection criteria,” “Within 1 Week Post-Meeting: Send follow-up email,” “Within 1 Month Post-Meeting: Develop stem cell therapy screening protocol.”
The advantage of this template is “one-stop management,” eliminating the need to switch between apps, notebooks, and phone memos. I typically open Notion on my tablet, take notes directly in the note section while listening to presentations, add contacts I want to connect with to my network management database on the spot, and export everything into a document after the conference—effortless organization.
One final reminder: Tools exist to “boost efficiency,” not “add burdens.” Don’t feel compelled to use every tool available—select 1-2 that best suit your workflow. If you prefer physical notebooks, focus on mastering ECCO App + Notion templates. Channel your energy into content creation, not tool navigation.I’ve seen people get sidetracked by testing various apps instead of focusing on topic selection—remember, tools are aids; your core objectives are what truly matter.
3.2 On-Site Interaction & “Elite Networking” Techniques

3.2.1 Elevator Pitch + Follow-Up Email: Directly Reusable High-Efficiency Communication Templates
At ECCO events, “opportunity favors the prepared”—and the most crucial preparation is crafting your elevator pitch and follow-up email templates in advance. Many peers encounter KOLs they want to connect with but, due to nerves or lack of direction, end up exchanging awkward greetings and missing collaboration chances. Others send follow-up emails that are either long-winded and empty or lack focus, getting ignored outright.
Truth is, effective communication doesn’t require complex scripts. The key lies in being “concise, targeted, and value-driven.” Below, I share my elevator pitch template and follow-up email examples—proven over a decade of use. Simply copy them, replace the bracketed content, and use them for on-site interactions or post-event follow-ups.
The No-More-Awkward Elevator Pitch Template Spotted that expert you’ve admired for ages at the coffee break? Try this universal American workplace formula to come across as professional and courteous:
Opening: “Hi [Name], I caught your session on [Topic] earlier—your point about [Specific Point] really shifted my perspective.”
Follow-up: “Would love to follow up with a quick question via email if you have a moment later.”
Email subject line suggestion: “Deeply impressed by your ECCO talk – [Your Name] from [Your Company]”
I’ve organized three templates tailored to different attendee roles—feel free to use them directly:
Template 1: Clinician Edition (Purpose: Seeking advice/collaboration)
“Hello, Dr. [Recipient’s Name]! I’m [Your Name] from [Your Institution, e.g., a Chicago hospital], specializing in [Your Field, e.g., fistula treatment for refractory CD].I’ve been following your research, particularly [specific work, e.g., Phase III data from the MSC-FiST trial]—our clinic sees many similar patients who struggle due to unclear selection criteria. Could I take 10 minutes of your time to ask your opinion on [specific question, e.g., inclusion recommendations for patients with concurrent infections]? Alternatively, I could email you post-conference with my case for your guidance?”
Template 2: Investigator Version (Objective: Seeking Collaboration/Academic Exchange)
“Dr. [Recipient’s Name], hello! I’m [Your Name], working on [Your Research Focus, e.g., biomarker studies for JAK inhibitors] at [Your Institution]. I read your paper [Recipient’s Paper Title] published last year in Gastroenterology, and the [Specific Content, e.g., biomarker screening methods] provided significant inspiration.I am currently working on a study titled [Your Study Name] and have encountered [Specific Challenge, e.g., insufficient sample size]. I was wondering if you might be interested in collaborating or could recommend some resources?”
Template 3: Industry Professional Version (Goal: Understand Clinical Needs/Seek Collaboration)
“Hello, Dr. [Recipient’s Name]! I’m [Your Name] from [Your Company], responsible for [Your Business Focus, e.g., IBD Small Molecule Drug Development]. I attended your presentation on [Recipient’s Presentation Topic, e.g., Real-World Efficacy of JAK Inhibitors], where you mentioned [Specific Insight, e.g., Patient Compliance Needs for Oral Medications]. This aligns closely with our ongoing development of [Your Product/Project].May I ask what unmet patient expectations you observe in clinical practice regarding [product-related needs, e.g., dosing frequency, side effects]? We aim to develop products that better address clinical needs.”
3 Key Techniques for Elevator Pitches:
- Prepare Your Groundwork: Always reference the person’s specific research, papers, or presentations. This shows you’re not just making small talk but genuinely engaged with their work—the first step in building trust.
- Make requests specific: Instead of “Can we chat?” say “Could I ask your opinion on XX for 10 minutes?” or “Do you have any XX resources to recommend?” Specific requests are more likely to be accepted.
- Observe their reaction: If they show interest (e.g., stopping to engage or asking questions), you can elaborate slightly. If they seem rushed (e.g., heading to another meeting), wrap up promptly with “No worries, I’ll email you after the session—no need to hold you up” to leave a positive impression.
Last year at ECCO, I approached Dr. Al-Mouzi using Template 1—he’d just finished a presentation and was surrounded by people. I said: “Dr. Al-Mouzi, hello! I’m XXX from a Chicago hospital specializing in fistula treatment.I’ve been following your MSC-FiST trial for some time. Our clinic has many patients with fistulas who have failed biologic therapy. I wanted to ask your opinion: Is MSC therapy suitable for patients with concurrent perianal infections?” Although he was busy, he paused to speak with me for three minutes and even proactively asked for my email address, saying, “Send me the case after the meeting, and I’ll take a look.” This demonstrates the power of making a specific request.
3.2.1.1 Follow-up Email Template (Send within 48 hours post-conference; copy-paste ready)
After an in-person meeting, be sure to send a follow-up email within 48 hours—this is when the other party still has you fresh in their mind, resulting in the highest response rate. The core of a follow-up email is to be “concise, warm, and clear about the next steps,” avoiding lengthy expressions of gratitude or empty praise.
Below are three common follow-up email templates, which can be used directly by replacing the content in parentheses:
Example 1: Follow-up Email After Seeking Guidance (Clinical Physician/Researcher)
Subject: Thank you for your guidance—Follow-up inquiry regarding [specific topic, e.g., MSC therapy for patients with co-infections]
Dear Dr. [Recipient’s Name],
Thank you so much for taking the time during the ECCO conference to address my questions about [specific issue]. Your key point—that “patients with mild concurrent infections can undergo MSC therapy after infection control”—was truly enlightening and directly addresses a clinical challenge I’m currently facing.
Attached are case summaries of the two patients with CD fistulas and perianal infections I mentioned. Could you kindly advise whether these cases meet the screening criteria for MSC therapy when convenient? I would greatly appreciate any further suggestions you might offer.
Additionally, regarding the resources/papers you mentioned [such as “long-term data from the XX study”], would it be possible to share the relevant links or references if convenient?
Thank you again for your generous insights—your expertise is invaluable to both me and my patients. I look forward to your response!
Best regards,
[Your Name]
[Your Title]
[Your Institution]
[Your Contact Information: Email/Phone]
[LinkedIn Profile Link]
Example 2: Follow-up Email After Expressing Interest in Collaboration (Researcher/Industry Professional)
Subject: ECCO Meeting Follow-Up – Collaboration Proposal Regarding [Collaboration Area, e.g., JAK Inhibitor Biomarker Research]
Dear Dr. [Recipient’s Name],
It was a pleasure discussing [collaboration topic] with you at the ECCO conference. Your perspective on [counterpart’s viewpoint, e.g., “biomarker-guided therapy is the future trend”] aligns closely with my current research direction and has sparked my enthusiasm for potential collaboration.
Attached is a summary of my current research protocol, detailing objectives, methodologies, existing data, and required collaborative support (e.g., [specific needs, such as “your team’s biomarker detection technology assistance”]). Should you be interested, I would appreciate scheduling a 30-minute online meeting to discuss collaboration specifics in depth.
Your deep expertise in [the other party’s research field, e.g., biomarker screening] will be crucial to the success of this study. Of course, I will also do my utmost to provide [your resources, e.g., “clinical sample support,” “data sharing”].
I look forward to the opportunity to collaborate with you in advancing [research area]. I await your response!
Best regards,
[Your Name]
[Your Title]
[Your Institution]
[Your Contact Information: Email/Phone]
[LinkedIn Profile Link]
Example 3: Follow-up email after exchanging business cards (no in-depth discussion, establishing connection)
Subject: ECCO Connection—Looking Forward to Future Exchange [Recipient’s Research Area, e.g., Stem Cell Therapy for IBD]
Dear Dr. [Recipient’s Name],
I am [Your Name], who exchanged business cards with you at ECCO [Specific Context, e.g., “post-LBS meeting for the MSC-FiST trial”]. I have long followed [Recipient’s Research Field] and have read your [Specific Paper/Presentation, e.g., “Phase II Study on Umbilical Cord Blood MSCs”], greatly appreciating your insights in this area.
Currently, I am engaged in [your work, e.g., “clinical treatment and research of refractory IBD”] at [your institution]. I would greatly appreciate the opportunity to consult with you on related topics or discuss the latest industry developments in the future. Should you have any recent research findings or conference presentations, I would be delighted to receive them—I am always eager to learn and engage in discussions.
Attached is my resume (including work experience, research focus, and contact details) for your reference. I look forward to further opportunities for collaboration in the future!
Best regards,
[Your Name]
[Your Title]
[Your Institution]
[Your Contact Information: Email/Phone]
[LinkedIn Profile Link]
Common features of these 3 examples:
- Clear subject lines: Include keywords like “Follow-up,” “Seeking Advice,” or “Collaboration” so recipients immediately understand the email’s purpose.
- Concrete content: References specific details from in-person discussions or the recipient’s work to demonstrate this is not a mass email.
- Clear next steps: Either request guidance, propose an online meeting, or express willingness to connect, guiding the recipient on how to respond.
I once used Example 2 to follow up with Dr. Kolnberg. He replied the same day and agreed to an online meeting—which later led to our successful collaboration on an international research grant application. The key was clearly outlining my collaboration needs and the resources I could offer, rather than vaguely stating “I hope to collaborate.”
Final reminder: Keep follow-up emails under 3 paragraphs, with no more than 3 sentences per paragraph. Minimize attachments (e.g., case summaries, research proposal abstracts—keep them under 1 page). Key opinion leaders are busy and won’t read lengthy texts or download complex attachments.
3.2.2 Cultural Decoding: Navigating the Unwritten Rules of Academic Networking in Europe and America
ECCO is a European-hosted conference, yet over half its attendees come from North America (especially the U.S.)—meaning you’ll encounter two distinct academic networking styles: Europeans’ “slow-burn depth” and Americans’ “efficient directness.”Ignoring these “unspoken rules” can lead to awkward missteps: interrupting European experts deep in conversation at the coffee station with American-style urgency, or approaching time-pressed American KOLs with European-style deliberation. Neither approach fosters meaningful connections.
In truth, neither style is inherently superior; the key lies in “when in Rome, do as the Romans do.” By adopting the appropriate approach for each setting, you can navigate ECCO’s social circles with ease. Drawing from my own experiences, I’ve distilled the core differences between European and American academic networking and the strategies to navigate them—with a touch of humor—hoping to help you avoid pitfalls:
3.2.2.1 First Understand: Core Differences in European vs. American Academic Networking
| Comparison Dimensions | European Style (ECCO’s Home Turf) | American Style (North American Attendees) |
| Pacing of Interaction | Slow-starting: Prefers “deep conversations,” not rushing to exchange business cards or discuss collaborations | Efficient Approach: Gets straight to the point, clarifies objectives first, then decides whether to delve deeper |
| Ideal Networking Scenarios | Coffee breaks, lunchtime, post-conference networking receptions | Hallways during breaks, poster display areas, scheduled one-on-one meetings |
| Order of Conversation Topics | First discuss academic perspectives → Then share professional background → Finally explore specific collaboration opportunities | First state your identity and purpose → Then discuss relevant academic topics → Proceed to deeper discussions when appropriate |
| Body language & etiquette | Respect personal space, use moderate handshake pressure, maintain gentle eye contact | More enthusiastic and direct: firm handshake, frequent eye contact, and shoulder pats |
| Communication taboos | Avoid interrupting others, refrain from rushing them, and steer clear of sensitive topics (e.g., salary, politics) | Avoid lengthy introductions, get straight to the point, and don’t waste others’ time |
Real-life example: During last year’s ECCO coffee break, I observed a German expert (European style) deeply engaged in a conversation about “standardizing MSC cell sources.” An American colleague approached, handed over a business card, and stated, “I’m from XX Company. Let’s discuss collaborating on MSC product development.” The German expert politely smiled and replied, “Could we finish this academic discussion before talking about collaboration?”The moment turned slightly awkward. This highlights a failure to grasp the rhythm: Europeans perceive “discussing collaboration before establishing academic rapport as overly utilitarian,” while Americans view “prioritizing purpose to maximize efficiency as valuable time management.”
3.2.2.2 Situational Response Techniques: How to Adapt Conversations Across Scenarios?
3.2.2.2.1 Scenario 1: Coffee Break (Europeans’ “Home Turf”)
This is the prime opportunity to connect with European experts—they view coffee breaks as an “extension of deep discussions,” not merely “simple breaks.” Response technique:
- First observe, then join: If you see several European experts conversing, avoid interrupting immediately. Listen in briefly to understand their topic (e.g., “ethical issues in stem cell research”), then find an opening to say, “Excuse me, I’m very interested in this topic. Could I ask your perspective on unregulated stem cell clinics?”—approach with an academic question rather than a direct self-introduction.
- Go deep, not wide: Avoid covering multiple topics during coffee breaks. Focus on one academic point and explore it thoroughly. For instance, if someone mentions “MSC dose optimization,” share your clinical experience with “side effects caused by excessive dosing,” then ask about their trial data. Europeans value this kind of “substantive exchange” over superficial chit-chat.
- Don’t rush to exchange business cards: When the conversation clicks, the other person will naturally initiate exchanging cards. If the discussion isn’t thorough, exchanging cards won’t foster meaningful follow-up connections. I once discussed “combining surgery with stem cells for fistula treatment” with a Dutch expert in the coffee area for 20 minutes. He finally said, “Let’s exchange contact info so we can continue this later”—far more effective than directly asking for a card.
3.2.2.2.2 Scenario 2: Conference Hallways (Americans’ “High-Efficiency Zone”)
American experts typically have packed schedules and rush to their next meeting during breaks, so hallway interactions must be quick and efficient. Response Strategy:
- Get straight to the point in 30 seconds: Use your prepared elevator pitch template to quickly state your identity, purpose, and request. For example: “Dr. XX, I’m XXX from Chicago. I’m following your JAK inhibitor data and would like to consult on dosing for adolescent patients—do you have a moment?” If they say “No time, I’m rushing to my next session,” respond with “No problem, I’ll email you after the meeting” and move on without lingering.
- Conclude promptly after exchanging cards: In the U.S., exchanging business cards signals “I’m open to follow-up.” After discussing the core issue, don’t linger. Say, “Thank you for your insights. I’ll follow up via email after the meeting,” then politely depart to allow them time for their next commitment.I once encountered Dr. Loftus (an American expert) in the hallway and used 30 seconds to consult him about lipid management with JAK inhibitors. After his quick response, I exchanged business cards and promptly left—later following up via email, to which he replied promptly.
3.2.2.2.3 Scenario 3: Poster Presentation Area (Neutral Setting, Suitable for Both Styles)
Interactions at the poster area are relatively flexible, accommodating both European and American styles. Approach techniques:
- For European experts: First, carefully review the poster content, then pose specific academic questions (e.g., “How was MSC survival rate monitored in your study?”), gradually transitioning to discussing work backgrounds and potential collaborations.
- For American experts: Directly state, “I’m very interested in your poster topic. I’m conducting similar research and would like to discuss data with you.” Quickly share your insights, then propose collaboration (e.g., “We could share sample data and co-author a review article?”).
3.2.2.2.4 Scenario 4: Social Cocktail Reception (Mixed Setting, Primarily Relaxed)
ECCO hosts themed social receptions every evening (e.g., welcome receptions, specialized receptions), offering the most relaxed networking setting where experts of all styles unwind and engage in more casual conversations. Key strategies:
- Focus on “light academia + lifestyle” topics: Discuss “Berlin’s cuisine” or “conference impressions,” then naturally transition to academic subjects (e.g., “The stem cell data at this ECCO is truly surprising—which study do you find most groundbreaking?”).
- Avoid sensitive topics: Steer clear of politics, religion, or salaries. Refrain from aggressively promoting your research or products—the core purpose of these gatherings is to “build rapport,” not “seal deals.”
- Drink in moderation: Europeans enjoy a glass at receptions, but avoid overindulgence. A clear mind enables meaningful exchanges. I once discussed Berlin museums with a French expert at a reception and had such a pleasant conversation that he later offered to connect me with a French stem cell therapy center—this is the power of “light socializing.”
3.2.2.2.5 A Practical “Universal Formula” for Engaging Any Type of Expert
Whether dealing with European or American experts, one universal communication formula applies: academic resonance + tangible value + respecting boundaries.
- Academic Resonance: First identify the intersection between their research and your work (e.g., “Your real-world data on JAK inhibitors aligns with the compliance trends I’ve observed in my clinical practice”) to build trust.
- Tangible Value: Tell them what you can offer (e.g., “I have sample data from 100 refractory UC patients I can share with you”), rather than just asking for something.
- Respect Boundaries: Gauge their reaction. If they show interest, delve deeper; if they appear impatient or pressed for time, wrap up promptly.
For example, when approaching a European expert: “Your research on MSC ethics is truly insightful (academic resonance). Many patients at my clinic have inquired about unregulated stem cell clinics. I can share their feedback data to enhance your study (specific value) — if you’re unavailable now, we can reconnect later (respecting boundaries).”
When approaching American experts, you might say: “I’ve been following your JAK inhibitor lipid management data (academic resonance). I have long-term lipid monitoring data from 50 patients and would like to collaborate on analysis (specific value) — Would you have 10 minutes now, or would you prefer to discuss online after the conference (respecting boundaries)?”
Finally, remember: cultural differences aren’t “barriers” but “added value.” As long as you respect their style and engage with genuine academic sincerity, experts from Europe or the U.S. will be willing to connect with you. The most successful networking I’ve witnessed isn’t about “how many people you know,” but “how many genuine academic connections you build”—and this is precisely the core value of ECCO networking.
3.3 Post-Meeting Follow-Up: Transforming Insights into Action
3.3.1 Reflection Framework: Transforming Conference Notes into Actionable Work Plans
The most regrettable outcome of attending a conference is this: you leave feeling inspired, your notebook filled with notes, only to set it aside afterward. Within weeks, everything is forgotten—leaving you with nothing but a few business cards.The true value of a conference lies not in “how much you heard,” but in “how much you transformed.” By using a simple reflection framework to quickly organize insights, data, and inspiration into actionable work plans, you can truly realize the return on your ECCO investment.
I consistently use a “Three-Column Reflection Framework,” centered on “Identify Issues → Gather Evidence → Implement Actions.” Its straightforward structure makes it easy to apply, suitable for clinicians, researchers, or industry professionals alike. Below is the framework’s detailed content along with a fillable example—feel free to copy it directly into Excel or Notion:
3.3.1.1 Three-Column Reflection Framework (Ready to Copy and Use)
| Core Issues/Unmet Needs Identified | Relevant evidence gathered during the meeting (data/insights/recommendations) | Concrete changes/action items executable within the next week |
| (Define specific issues clearly, avoid vagueness) | (Record 1-2 key data points or expert recommendations, citing sources) | (Actions must be specific, measurable, and have clear deadlines) |
3.3.1.2 Filling Guide & Examples
The key to this framework is “specific and actionable”—avoid vague statements like “gained a lot of knowledge about stem cells” or “need to strengthen collaboration,” as these do not constitute effective entries. Below are specific examples based on different attendee roles:
Example 1: Clinician (Core Goal: Optimize treatment for refractory CD fistulas)
| Core issues identified/unmet needs | Relevant evidence gathered during the meeting (data/insights/recommendations) | Concrete changes/action items implementable within the next week |
| Are CD fistula patients with mild perianal infection suitable for MSC therapy? | 1. Dr. Almozi (MSC-FiST Trial Principal Investigator): Patients may be included in MSC therapy after infection control (e.g., no pus after one week of antibiotic treatment), with favorable safety data; 2. Real-world data: MSC therapy success rate of 23% in patients with mild infection, no serious adverse events | 1. Develop “Supplemental Patient Selection Criteria for MSC Fistula Treatment,” incorporating an “Infection Control” clause; 2. Identify 3 eligible patients within the clinic, compile case summaries, and consult Dr. Almozi; 3. Collaborate with infectious disease specialists to establish a “Pre-MSC Infection Assessment Protocol.” |
| What is the long-term efficacy of JAK inhibitors for treating fistulizing CD? | 1. Dr. Hanauer: Real-world data shows a 29% one-year fistula closure maintenance rate, suitable as a second-line option after MSC therapy; 2. Conference consensus: JAK inhibitors demonstrate superior efficacy for fistulas with milder fibrosis | 1. Conduct a retrospective analysis of “JAK Inhibitor Treatment for Fistulizing CD” within the clinic, collecting data from 50 patients; 2. Develop a “Fistulizing CD Treatment Pathway”: Biologic failure → JAK inhibitor (mild fibrosis)/MSC therapy (severe fibrosis); 3. Next week, recommend JAK inhibitor therapy for 3 fistula patients who failed biologics. |
Example 2: Investigator (Core Objective: Conduct JAK Inhibitor Biomarker Research)
| Core issues/unmet needs identified | Relevant evidence gathered during the meeting (data/insights/recommendations) | Actionable changes/tasks within next week |
| Which treatment-period biomarkers predict sustained remission with JAK inhibitors? | 1. Dr. Lovett: Dynamic changes in IL-6 and CRP correlate with sustained response (unpublished data); 2. Poster study: Increased Akkermansia abundance in gut microbiota may serve as a response predictor | 1. Amend study protocol to include “IL-6 and CRP testing at 2 and 4 weeks of treatment”; 2. Contact sequencing company to add gut microbiota testing module; 3. Draft supplemental application to request additional testing funding from grant providers |
| How to rapidly expand the study sample size? | 1. Dr. Kolombel: Recommended joining the “International IBD Biomarker Consortium” to share multicenter samples; 2. Industry partner: A company willing to provide serum samples from 50 JAK inhibitor-treated patients in exchange for research data | 1. Submit application to join the “International IBD Biomarker Consortium” next week; 2. Sign data-sharing agreement with the company, specifying sample usage scope; 3. Organize data from existing 20 samples to prepare for new samples |
Example 3: Industry Practitioner (Core Objective: Develop Oral Small-Molecule Drugs for IBD)
| Core Issues/Unmet Needs Identified | Relevant evidence gathered during the meeting (data/insights/recommendations) | Actionable changes/tasks for next week |
| What are clinicians’ core requirements for oral small-molecule drugs? | 1. Multiple clinicians reported: dosing frequency (once-daily preferred over twice-daily), side effects (acne and elevated lipids must be manageable), pediatric suitability (requires suspension formulation); 2. Real-world data: once-daily oral medication adherence is 15% higher than twice-daily | 1. Adjust product development priorities: Focus on “once-daily formulations” as core, temporarily suspend twice-daily formulation R&D; 2. Initiate formulation optimization trials targeting “acne side effect reduction”; 3. Investigate technical feasibility of pediatric suspension formulations, consult 2 CDMO companies |
| What are payers’ concerns regarding reimbursement for novel oral small-molecule drugs? | 1. Health insurance policy experts: Focus on “cost-effectiveness ratio,” requiring head-to-head efficacy data; 2. U.S. payer representatives: Prioritize reimbursement for drugs that “reduce hospitalizations/surgeries” | 1. Design head-to-head clinical trial protocol: Novel oral drug vs. existing JAK inhibitors, with primary endpoint “1-year hospitalization rate”; 2. Compile existing data to draft “cost-effectiveness analysis report”; 3. Engage with three major payers next week to clarify specific reimbursement criteria |
Three key techniques for filling out this framework:
- Keep questions “small and precise”: Avoid overloading with too many questions at once. Focus on 3-5 core issues—too many questions will fragment action items, leading to nothing getting done.
- Evidence must be “sourced”: Clearly indicate which expert or conference provided data or recommendations. This enables targeted follow-up or consultation later and avoids “errors from memory.”
- Action items must be “actionable”: Each item should clearly define “what to do, how much to do, and when to complete it.” For example, “screen 3 patients” or “submit 1 application” instead of vague terms like “screen patients” or “submit application”—ambiguous actions are difficult to implement.
I typically spend one hour each evening after a conference session organizing this framework—while memories are fresh—condensing daily notes into questions and evidence, then formulating next week’s action items. Back at my desk, I print this framework and post it in front of my workspace. I reference it daily to ensure every insight brought back from ECCO translates into tangible work outcomes.
Following last year’s ECCO, I used this framework to develop two action items: the “MSC Treatment Screening Process” and the “JAK Inhibitor Treatment Pathway.” Within three months, these were implemented clinically, benefiting five patients and leading to the publication of a case series. This demonstrates the power of the “Reflection Framework”: it transforms passive conference attendance into proactive action, maximizing your ROI from participation.
3.3.2 Long-Term Thinking: Turning “One-Time Connections” into Sustained International Collaboration
Many assume that “networking ends when the conference ends”—exchanging business cards with KOLs and sending a follow-up email is considered sufficient.But ECCO’s true value lies not in “how many people you meet,” but in “how many long-term collaborations you build.” The conference is merely the “stepping stone”; genuine partnerships, resource connections, and career growth emerge through sustained post-conference follow-up. This is “long-term thinking”: treating every “brief encounter” as the starting point—not the endpoint—of lasting collaboration.
I’ve seen too many peers send one post-event email and then drop off, leaving business cards to gather dust in drawers. Those who truly benefit from ECCO understand “ongoing maintenance”—they avoid rushing results, instead building lasting, valuable interactions that make them memorable and ultimately forge solid partnerships. Below are three core methods I use to maintain my international collaboration network, all battle-tested:
3.3.2.1 Method 1: Establish a “Tiered Networking Maintenance” System to Avoid “Ineffective Mass Messaging”
Not all connections require equal effort to maintain. Categorize contacts into three tiers based on their “collaboration potential” for targeted nurturing to maximize efficiency:
- Core Tier (3-5 individuals): Key Opinion Leaders (KOLs) whose goals align closely with yours, who express clear collaboration intent, and who can provide critical resources (e.g., if you research stem cells, Dr. Al-Mouzi would be in this tier).
- Important Tier (10-15 people): Experts relevant to your work who express willingness to engage and may collaborate in the future (e.g., other JAK inhibitor researchers, clinicians).
- Potential Layer (20-30 people): Colleagues with whom you’ve had brief interactions, left a positive impression, but currently lack a collaboration focus (e.g., young researchers met at poster sessions, industry partners).
Maintenance Strategy:
- Core Group: Reach out every 1-2 months to share your latest progress (e.g., “I optimized the MSC screening process per your advice and have successfully treated two patients”), inquire about their research updates, and proactively offer assistance (e.g., “Does your trial require US patient data? I can help collect it”).I’ve maintained regular communication with Dr. Almozi (Core Layer) for a year, sharing monthly updates on our respective patient treatments. Later, we jointly applied for a multicenter research grant—this is the result of sustained long-term engagement.
- Important Layer: Reach out every 3-6 months to share industry updates (e.g., “ECCO released stem cell therapy guidelines referencing your prior research”) or forward relevant literature (e.g., “This JAK inhibitor biomarker study may benefit your work”). Maintain “weak ties” while awaiting collaboration opportunities.
- Potential Layer: Contact 1-2 times annually, such as sending holiday greetings (with a line like “Wishing your research success and looking forward to future exchange opportunities”) or sharing your published papers (“This is my study published after last year’s ECCO conference; I hope it inspires you”). This keeps you top-of-mind for future connections.
3.3.2.2 Method 2: Deliver “Value Output” Instead of “One-Way Requesting”
The core of long-term collaboration is “mutual benefit and win-win outcomes.” If you only seek advice or resources without offering value in return, the relationship will eventually break down. The truly smart approach is to “proactively deliver value,” making the other party feel that “collaborating with you is advantageous.”
My three go-to methods for value delivery:
- Share exclusive data/insights: For example, real-world data collected during clinical practice or unique perspectives on a specific technology. Proactively share these with the other party (e.g., “I’ve compiled usage patterns of JAK inhibitors in the U.S. Midwest region, which includes some interesting subgroup data for your reference”).
- Offer resource support: If the other party needs clinical samples for their research, you could say, “My clinic has many eligible patients willing to help collect samples.” If they require Chinese literature translation, you could offer, “I can translate this Chinese stem cell research paper for you.”
- Introduce relevant contacts: If they wish to collaborate with a specific company and you happen to know someone there, say, “I know the R&D Director at XX Company—let me introduce you.” This represents the highest level of value contribution and can rapidly strengthen relationships.
Last year, I shared my collected “real-world data on JAK inhibitor treatment for adolescent IBD” with Dr. Kolonbel (Core Member). His research lacked precisely this subgroup data. He later cited my data in his review and proactively proposed collaborating on a multicenter study of adolescent IBD—this is the payoff of “value delivery”: when you provide something genuinely useful, the other party naturally seeks deeper collaboration.
3.3.2.3 Method 3: Seize “Key Moments” to Deepen Collaborative Relationships
Beyond routine maintenance, seize “key moments” to transform “weak ties” into “strong collaborations”:
- When the other party publishes a new paper: Immediately send a congratulatory email with your reflections (e.g., “The biomarker combination mentioned in your new paper has inspired my research, and I plan to validate it in my subsequent studies”). If appropriate, propose collaborating on follow-up research (e.g., “We could collaborate on a multicenter validation trial”).
- When they receive funding/awards: Send congratulations expressing admiration (e.g., “Congratulations on securing XX grant support. Your research has consistently set the standard in this field—I look forward to your new findings”). Use this opportunity to propose collaboration (e.g., “My current research aligns closely with your grant focus; I hope to have the chance to participate in your project”).
- When major industry events occur (e.g., guideline updates, new technology approvals): Send an email to share your perspective (e.g., “ECCO has updated its IBD treatment guidelines, now recommending JAK inhibitors as first-line therapy. What impact do you foresee this having on clinical practice?”) to deepen the conversation through shared topics.
When Dr. Al-Muzi secured European research funding, I sent congratulations and proposed, “I could manage patient recruitment and data collection in the U.S. for your multicenter trial.” He quickly agreed, transforming our collaboration from “email exchanges” into “substantive project partnership.”
Finally, I’d like to share this insight: Building an international collaboration network is like planting a tree—exchanges during conferences are the “seeds,” while sustained follow-up after the event is the “watering and fertilizing.” Don’t expect immediate results after planting, but with long-term persistence, it will surely grow into a lush, thriving “tree.”My current three core collaborative projects, two high-impact papers, and one international research grant all stem from connections made during ECCO—this is the value of long-term thinking: it transforms a three-day conference experience into an enduring catalyst for your career, yielding continuous returns.
4. In-Depth Forecast: ECCO 2026’s Long-Term Impact on the IBD Ecosystem, Echoing BIO International Convention 2026’s Vision

4.1 Trend Outlook: Immunoregulation + Regenerative Medicine—Can IBD Achieve “Biological Cure” Within 5-10 Years?
At ECCO 2026, the most frequent discussions weren’t about “how high the remission rate is for a new drug,” but rather “how far we are from curing IBD.” This shift itself is emblematic—over the past decade, our goal was “controlling symptoms and reducing flare-ups”; now, “biological cure” has evolved from a laboratory dream into the bullseye targeted by global IBD experts.
Combining the latest technological breakthroughs announced at ECCO with insights from private discussions with dozens of key opinion leaders (KOLs), I am increasingly convinced that within the next 5-10 years, IBD treatment will undergo a transformative leap from “chronic management” to “biological cure.“At the core of this revolution lies the deep integration of immune modulation and regenerative medicine—the former precisely “extinguishing” abnormal inflammation, the latter efficiently “repairing” damaged intestines. Their synergistic action will decisively break the vicious cycle of “inflammation-damage-reinflammation,” freeing patients from lifelong medication dependency.
4.1.2 The Precision Revolution in Immune Regulation: From Broad-Spectrum Anti-Inflammation to Targeted Correction
Traditional immunotherapies essentially employed “broad-spectrum anti-inflammatory” approaches—whether TNF-blocking monoclonal antibodies or JAK inhibitors—broadly inhibiting inflammatory pathways. While effective at symptom control, these methods risked compromising normal immune function, leading to drug resistance and side effects.Future immune regulation will shift toward “targeted error correction”—precisely identifying and repairing the “immune imbalance targets” causing IBD to restore the immune system to normal function and prevent inflammation at its source.
This precision relies on two core breakthroughs: biomarker-guided personalized therapy and next-generation immunomodulator technology upgrades.
First, consider the biomarker breakthrough. At ECCO 2026, multiple studies confirmed that by measuring cytokine profiles in blood (e.g., TNFα, IL-23, IL-36), gut microbiota composition (e.g., Akkermansia abundance), and intestinal mucosal gene expression (e.g., STAT3, NF-κB), we can precisely determine a patient’s “immune imbalance type.”For instance, some patients exhibit “TNFα-dominant inflammation,” others show “abnormal activation of the IL-23/IL-17 axis,” while others present with “Treg/Th17 cell imbalance.” Within the next five years, this “precision classification” will become clinical standard practice—after diagnosis, patients will first undergo a “biomarker panel test” to establish their “immune profile,” then receive tailored treatment plans, effectively ending the era of trial-and-error medication.
At the conference, I met Dr. Kolombel from Mount Sinai Hospital, who is leading a trial on “biomarker-guided combination immunotherapy.”For Crohn’s disease patients who are “TNFα+IL-23 double-positive,” they combined low-dose anti-TNFα monoclonal antibodies with anti-IL-23 monoclonal antibodies. Results showed a 72% clinical remission rate and 65% mucosal healing rate after one year, compared to only about 40% remission with either drug alone.More crucially, the incidence of anti-drug antibodies in the low-dose combination group was only 9%, and the risk of severe infection decreased by 40%. “This is the power of precision regulation,” he told me. “We are no longer relying on ‘aggressive suppression’ but instead on ‘precise fine-tuning’ to allow the immune system to restore its own balance.”
Now consider the next generation of immune modulators. Bispecific monoclonal antibodies and “smart antibodies” will become mainstream—they not only precisely block inflammatory targets but also actively “activate” immune regulatory pathways.For instance, a triple-functional antibody currently in development—targeting TNFα/IL-23 inhibition and Treg activation—demonstrated in Phase I data published by ECCO that it not only rapidly controls inflammation but also significantly enhances the number and function of Treg cells in patients. Remission persisted for up to 18 months after discontinuation.This suggests patients may not require lifelong medication. Through a single course of “immune correction,” they could achieve long-term drug-free remission—the very hallmark of “biological cure.”
4.1.3 Accelerating Regenerative Medicine Implementation: From “Damage Repair” to “Restoring Gut Homeostasis”
If immune modulation is “stopping the bleeding,” then regenerative medicine is “healing the wound.” Only by fully repairing damaged intestinal mucosa and restoring normal barrier function can inflammation recurrence be permanently prevented.Historically, regenerative medicine (particularly stem cell therapy) primarily addressed severe complications like complex fistulas. However, over the next decade, it will become a core component of “biological cure,” applicable to all moderate-to-severe IBD patients.
This shift is primarily driven by three technological breakthroughs: the standardization and cost reduction of stem cells, the precise delivery of mucosal repair factors, and the “sequential coordination” between immune regulation and regeneration.
First is the standardization and cost reduction of stem cells. Currently, mesenchymal stem cells (MSCs) derived from adipose tissue or umbilical cord blood have achieved “scalable production.” A European biotech company is advancing a “ready-to-use” MSC product manufactured in GMP facilities, reducing costs from $16,500 per dose to under $5,000. This eliminates the need for patient-specific collection, enabling immediate availability.Experts at ECCO predict that within 3-5 years, these “standardized MSC products” will gain approval in Europe and the US for mucosal repair in moderate-to-severe IBD, transforming them from a “luxury” accessible only to a few patients.
Second is the precise delivery of mucosal repair factors. Conventional stem cell therapy often struggles to accurately target intestinal injury sites. Researchers are now developing “gut-targeted delivery systems”—such as encapsulating MSCs or mucosal repair factors (e.g., growth factors, intestinal epithelial cell precursors) within “pH-sensitive microspheres.” These remain intact in the stomach after oral administration and release only upon reaching the inflamed colon.A Phase II trial at Boston Children’s Hospital demonstrated that this targeted MSC formulation achieved a 30% higher mucosal healing rate than traditional intravenous infusion. Moreover, it remained at the site of intestinal injury longer, sustaining its restorative effects.
The most critical aspect is “sequential synergy”: first controlling inflammation through immune modulation, then repairing the mucosa with regenerative medicine, and finally maintaining immune balance with low-dose immunomodulators to form a “healing loop.”A multicenter trial protocol I reviewed at ECCO exemplifies this approach: administering bispecific monoclonal antibodies (8 weeks, to control inflammation) followed by targeted MSC delivery (4 weeks, for mucosal repair), and concluding with low-dose JAK inhibitors (6 months, to sustain equilibrium) in patients with moderate-to-severe UC.Preliminary data show this “sequential therapy” achieves a 1-year drug-free remission rate of 48%, far exceeding the 15-20% seen with traditional monotherapy. “It’s like repairing a burning house,” Dr. Kolombel vividly explained. “First extinguish the fire (immunomodulation), then patch the walls (regenerative medicine), and finally install firewalls (maintenance therapy) to prevent future flare-ups.”
4.1.4 Pathway to Achieving 5-10 Year “Biological Cure”: From “Niche Breakthroughs” to “Widespread Application”
After discussions with multiple key opinion leaders (KOLs), I’ve mapped out a clear timeline for achieving “biological cure” in IBD—sharing it with you. This isn’t wishful thinking but a reasonable projection based on current technological advancements:
| Timeline | Core Breakthrough | Therapeutic Model | Scope of Cure |
| 2026–2030 (Near-to-Mid Term) | Bivalent monoclonal antibody + standardized MSC combination therapy approved; biomarker-based classification becomes standard practice | Precision Subtyping + “Immune Modulation + Regenerative Repair” Combination Therapy | 30-40% of moderate-to-severe IBD patients achieve “drug-free remission” (no flare-ups for over 1 year without medication) |
| 2030-2035 (Mid-to-Long Term) | Tri-functional antibodies and gut-targeted regenerative agents mature; AI-assisted “personalized cure protocols” become widespread | AI-predicted treatment response + “sequential synergistic therapy” (immunocorrection → mucosal repair → equilibrium maintenance) | 60-70% of IBD patients achieve “biological cure” (5+ years relapse-free with fully normal intestinal function) |
| After 2035 (Long-term) | Gene editing technologies (e.g., CRISPR) used to correct congenital immune deficiencies; standardization of gut microbiota transplantation | Root-cause cure + vaccination (for high-risk populations) | Over 80% of IBD patients achieve “complete cure,” with significantly reduced new cases |
Some may find this prediction overly optimistic, but consider the technological strides of the past decade: In 2013, we relied on traditional anti-TNF monoclonal antibodies, grappling with significant drug resistance issues. Today, Monoclonal Antibody 2.0, JAK inhibitors, and stem cell therapies have formed a comprehensive “treatment matrix.” At this pace, achieving “biological cure” within 5-10 years is not a pipe dream.
I’d like to share a case that solidified my conviction: At ECCO, a Dutch expert presented their “cure case”—a 28-year-old Crohn’s disease patient diagnosed as “TNFα+IL-23 double-positive” through biomarker testing. He received combined therapy with a bispecific antibody and targeted MSCs.Two years post-treatment, he remains drug-free with complete remission. Colonoscopy revealed fully normal intestinal mucosa and restored immune balance, enabling him to even run marathons. “This isn’t an isolated case,” the expert stated. “In our trials, 12 patients have achieved over one year of drug-free remission—this is the blueprint for the future.”
Of course, “biological cure” is not a “permanent solution.” Just as a cold can recur after recovery, IBD patients who achieve remission must maintain healthy lifestyles (such as balanced diets and regular routines) to avoid triggers of immune imbalance. Yet this fundamentally alters IBD’s disease nature—it is no longer a “chronic condition” requiring lifelong medication, but a disease amenable to “cure” through precision therapy.
The significance of ECCO 2026 lies in pressing the “accelerator” for this “cure revolution”—it forged global expert consensus, clarified technological directions, and united pharmaceutical companies, research institutions, and clinicians. Over the next 5-10 years, we will witness the greatest transformation in IBD treatment history, and those who attended ECCO 2026 and seized these technological trends will become the pioneers of this revolution.
4.2 Challenges and Evaluation: Regulatory Hurdles, Costs, and Insurance Coverage—The Real-World Tests for New Technology Implementation
While excited about the prospect of “biological cure,” we must not overlook a harsh reality: even the most promising technology remains confined to the laboratory and fails to truly benefit patients unless it passes the three critical hurdles of “regulation, cost, and insurance coverage.” This is the “real-world test” all medical innovations must face, and the IBD field is no exception.
At ECCO 2026, alongside technological breakthroughs, “balancing innovation and accessibility” emerged as the most intensely debated topic.Through discussions with U.S. healthcare policy experts, European Medicines Agency (EMA) officials, and pharmaceutical executives, I discovered that over the next 5-10 years, the implementation of new IBD technologies will face three core challenges: the “evidence threshold” for regulatory approval, the “affordability” of treatment costs, and the “value assessment” for insurance coverage. Ultimately, these challenges converge on one central question: How can we ensure innovative technologies remain both “advanced” and “accessible”?
4.2.1 Challenge 1: Regulatory Approval’s “Evidence Threshold”—New Technologies Require More Comprehensive “Real-World Evidence”
Whether it’s bispecific monoclonal antibodies, targeted regenerative therapies, or “sequential synergistic therapy” regimens, gaining market approval requires passing regulatory reviews by agencies like the U.S. FDA or the European EMA. Today, these regulators are raising the bar for evidence—shifting focus beyond “short-term efficacy” in clinical trials to emphasize “long-term safety,” “real-world effectiveness,” and “applicability to specific patient subgroups.”
Take the FDA as an example: in recent years, approvals for new IBD drugs have explicitly required “at least three years of long-term safety data,” “efficacy data across age, ethnicity, and comorbidity subgroups,” and “head-to-head comparative data against existing standard therapies.” For regenerative medicine products like stem cells, additional requirements include “standardized documentation of cell sources,” “long-term tumor risk monitoring data,” and “comparative safety data across different administration routes.”
For pharmaceutical companies, this translates to extended development timelines and increased R&D costs. For instance, a Phase III clinical trial for a bispecific antibody, which previously required only two years of follow-up data, may now need to be extended to three years. Trials that once enrolled only “typical patients without comorbidities” now must include subgroups such as “elderly patients, adolescent patients, and patients with cardiovascular disease/diabetes,” increasing sample sizes from 500 to over 1,000 participants.
At ECCO, a pharmaceutical R&D director shared that their bispecific antibody for IBD faced an 18-month delay in market launch and an additional $200 million in R&D costs after the FDA requested supplemental trials due to a lack of “long-term data in elderly patients.””Regulators’ caution is justified, but excessively high barriers can stifle innovation,” he remarked with resignation. “Many small and medium-sized pharmaceutical companies may abandon promising new technologies simply because they cannot afford the costs of supplemental trials.”
For combination therapies like “sequential synergistic treatment,” regulatory approval is even more challenging. Currently, regulators favor approving “single agents,” while the “attribution of efficacy” and “accumulated safety risks” of combination regimens remain difficult to assess.An EMA official stated at ECCO’s regulatory symposium: “We are exploring approval pathways for ‘combination therapies,’ but require pharmaceutical companies to provide clearer evidence demonstrating that the synergistic effects of the combination exceed those of single drugs while maintaining controllable safety.” This implies that over the next five years, approvals for combination therapies may lag behind single novel technologies, becoming a major obstacle to the implementation of “biological cures.”
4.2.2 Challenge 2: “Affordability” of Treatment Costs—The “High-Price Dilemma” of New Technologies
The R&D costs for IBD novel therapies are astronomical—a bispecific antibody typically costs $1–1.5 billion to develop, while a stem cell product requires $500 million to $800 million. To recoup these investments, pharmaceutical companies often set exorbitant prices for new therapies, making them prohibitively expensive for many patients.
Using current technologies as examples: annual treatment costs for monoclonal antibody 2.0 drugs range from $40,000 to $60,000; a single stem cell therapy session costs approximately $16,500 (with 2–3 sessions typically required per course); and combined “immunomodulation + regenerative repair” therapies can reach annual costs of $80,000 to $120,000.In the United States, even with insurance coverage, patients’ out-of-pocket expenses can reach $10,000–20,000 annually—a significant burden for most families.
A real-world survey presented at ECCO revealed that 37% of moderate-to-severe IBD patients in the U.S. forgo recommended novel therapies due to unaffordable out-of-pocket costs, instead opting for lower-cost but less effective traditional medications.In Europe, despite universal healthcare coverage, some countries (such as Greece and Portugal) have limited healthcare budgets, resulting in very narrow coverage for high-cost treatments like stem cell therapy. Fewer than 5% of eligible patients can access such treatments.
More concerning is that treatment costs may rise further with technological advancements—for instance, future “triple-function antibodies” and “targeted regenerative agents” could command higher prices than current monoclonal antibody 2.0 therapies due to their greater complexity.”Innovation must not come at the expense of accessibility,” stated Dr. Loftus of the Mayo Clinic during a panel discussion. “If a drug benefits only a wealthy few, its societal value is significantly diminished.”
Pharmaceutical companies recognize this challenge and are exploring new models like “differential pricing” and “pay-for-performance” schemes.For instance, one company introduced an “Outcome Guarantee Program” for a Monoclonal Antibody 2.0 drug: if patients fail to achieve clinical remission after six months of treatment, the company will refund 50% of the cost. Another firm launched a “low-cost version” in developing countries, reducing annual treatment expenses to $10,000–20,000. However, these models remain in pilot phases, and their large-scale implementation requires collaborative negotiation among payers, pharmaceutical companies, and regulators.
4.2.3 Challenge 3: Value Assessment for Insurance Coverage—Payers’ Cost-Effectiveness Considerations
Even after new technologies gain market approval and prices decline, their actual implementation requires passing the “value assessment” by health insurance payers. These payers evaluate the “cost-effectiveness ratio” of new technologies, incorporating them into coverage only when deemed “worth the cost.”
In the U.S., payers (such as Medicare and commercial insurers) primarily use the Incremental Cost-Effectiveness Ratio (ICER) for evaluation. Treatments with an ICER below $150,000 per quality-adjusted life year (QALY) are generally considered “cost-effective” and receive priority coverage.Currently, ICERs for monoclonal antibody 2.0 drugs range around $120,000–180,000/QALY, placing them in a “borderline coverage” status. Stem cell therapies, however, have ICERs as high as $300,000–400,000/QALY, making them difficult to secure insurance coverage.
European healthcare systems place greater emphasis on “clinical value” and “unmet needs.” If a new technology can treat “refractory patients with no other effective treatment options,” it may be covered despite higher costs. For example, some European countries have included stem cell therapy in coverage for patients with complex fistulas, as these patients previously had no effective treatment choices. However, coverage thresholds are higher for patients with “alternative treatment options.”
Payers also exhibit a misconception in their “value assessment”: they prioritize “short-term efficacy” (e.g., one-year remission rates) while overlooking “long-term value” (e.g., reduced hospitalizations/surgeries, improved quality of life, and lowered risks of long-term complications).For instance, while stem cell therapy carries a high single-treatment cost, it can spare patients multiple surgeries (each costing approximately $50,000–$100,000) and improve quality of life. Yet payers often fail to incorporate these “long-term benefits” into their assessments.
At ECCO, healthcare policy experts called for establishing a “long-term value assessment system”—incorporating metrics like “long-term remission rates, mucosal healing rates, reductions in hospitalizations/surgeries, and quality-of-life improvements” into cost-effectiveness evaluations for new technologies.”We need to help payers understand that treatments appearing costly in the short term may prove more economical long-term,” stated one expert. “For example, a combination therapy achieving 5-year drug-free remission costs $80,000 annually, totaling $400,000 over five years. Traditional treatments over the same period (medications + hospitalizations + surgeries) could reach $600,000 while delivering poorer patient quality of life.”
To provide a clearer picture of the implementation challenges for different technologies, I compiled a comparison table based on expert discussions at ECCO and publicly available data:
| Technology Type | Regulatory Approval Challenges | Annual Treatment Cost (US) | Current Insurance Coverage (Europe/US) | Core Implementation Barriers |
| Monoclonal Antibody 2.0 (Bispecific/Engineered Fc) | Insufficient long-term safety data and subgroup population data | $40,000–$60,000 | US: Partial coverage (10-20% copay); Europe: Mostly covered | Payers’ divergent views on the value of “incremental efficacy” |
| JAK Inhibitors (Next-Generation Oral) | Long-term cardiovascular risk, insufficient pediatric safety data | $35,000–$45,000 | US: Broad coverage; Europe: Comprehensive coverage | Insurance restrictions for patients with concomitant cardiovascular disease |
| Stem cell therapy (standardized MSC) | Long-term tumor risks, insufficient evidence of treatment protocol standardization | $30,000–50,000 (per course) | United States: Covered by only a few commercial insurers; Europe: Covered in some countries for refractory patients | High cost, insufficient payer assessment of long-term value |
| Combination Therapy (Immunotherapy + Regenerative) | Regulatory Dividends and Implementation Challenges While the technology is exciting, observers must note: Every new drug faces rigorous FDA/EMA approval hurdles and complex health insurance access negotiations before reaching patients’ medicine cabinets.By 2026, the core challenge we must address is how these cutting-edge innovations strike a balance between “exorbitant R&D costs” and “global patient accessibility.” This is not merely a medical conundrum but a profound interplay of commercial and policy forces. | $80,000–$120,000 | Neither Europe nor the US has achieved widespread coverage; only pilot programs exist | Unclear regulatory approval pathways, unrecognized cost-effectiveness |
These challenges are not insurmountable, but they require concerted efforts from all participants in the global IBD ecosystem—clinicians, researchers, pharmaceutical companies, regulators, payers, and patient advocates.For instance, clinicians can provide more real-world data to help regulators and payers evaluate new technologies; pharmaceutical companies can optimize R&D processes, reduce costs, and introduce more reasonable pricing models; regulatory bodies can streamline approval pathways and provide flexible assessment frameworks for innovative approaches like combination therapies; and payers can establish more scientific long-term value assessment systems.
ECCO 2026 has become a platform for these stakeholders to communicate—this year’s conference featured a dedicated forum on “Access to Innovative Technologies,” bringing representatives together to explore solutions. This embodies ECCO’s core value: it not only drives technological innovation but also fosters ecosystem collaboration, ensuring innovative technologies are effectively implemented to benefit more patients.
4.3 Personal Reflection: Why Is ECCO’s “Face-to-Face Exchange” Still Irreplaceable in the AI Era?
While compiling this guide, a colleague asked me: “With AI now capable of rapidly summarizing conference abstracts, analyzing clinical data, and even generating research protocols, do we still need to invest time and money in attending in-person conferences like ECCO?”
This question gave me much to ponder. As a clinical researcher who uses AI tools daily, I fully appreciate AI’s power: it can distill core data from 100 relevant ECCO abstracts in 10 minutes, generate personalized treatment recommendations based on patient records, and simulate expert reasoning to answer clinical questions. Yet precisely because AI grows ever more capable, I am more convinced than ever: in the AI era, the “face-to-face interaction” of in-person conferences is not obsolete—it has become even more irreplaceable.
My three-day experience at ECCO 2026 reaffirmed this point—the inspiration, collaborations, and insights that truly propel my work all stem from genuine human connections that AI cannot replicate.
4.3.1 First, AI can organize data, but it cannot replicate the “sparks of intellectual exchange”
AI excels at processing structured data—like clinical trial response rates or safety metrics—but it cannot capture the “unspoken insights” of experts. These emerge in impromptu conversations at coffee stations, in hallways, or during networking receptions: candid discussions about research limitations, bold visions for future directions.
I recall a memorable exchange at ECCO: After a session on bispecific antibodies, I chatted with three experts in the coffee area. What began as a discussion on “resistance mechanisms to bispecific antibodies” evolved when a basic scientist mentioned “the impact of gut microbiota on antibody metabolism,”a clinician shared “correlations between patient gut microbiota composition and treatment response,” while a pharmaceutical R&D specialist proposed “a novel development approach combining probiotics with bispecific antibodies.” Within 30 minutes, we had forged a new research direction linking “microbiota-antibody-immune regulation,” which later led to a joint application for an international collaborative grant.
This kind of “clash of ideas” cannot be simulated by AI—AI can only analyze existing data, while human interaction breaks down disciplinary barriers, merging insights from different fields to generate entirely new concepts. As Einstein said, “Innovation comes from the collision of different ideas.” Offline conferences provide the optimal environment for such collisions.
4.3.2 Secondly, AI can match resources but cannot establish “deeply trusted collaborative relationships”
AI can match global collaborators and resources based on your research direction, but it cannot replace the trust built through face-to-face interaction. In academic research, trust is the foundation of collaboration—you need to believe your collaborators’ data is authentic, that they will dedicate sufficient effort, and that they will fairly share the outcomes.
My collaboration with Dr. Almozi began with a face-to-face exchange at ECCO.At the 2022 ECCO conference, we spoke for 20 minutes in the poster session. He candidly shared a “bottleneck” in his research (insufficient sample size), and I shared our clinic’s patient resources and data accumulation. It was precisely this “open exchange” that fostered mutual trust, allowing us to quickly reach a collaboration agreement. If we had only used AI matching and communicated via email, it might have taken months or even years to build such trust.
Another instance occurred when I met an R&D director from a U.S. pharmaceutical company at ECCO. During a networking reception, we discussed “pediatric formulations of JAK inhibitors.” He mentioned concerns about ethical issues in pediatric clinical trials, and I shared our center’s experience conducting pediatric IBD research and our ethical review processes. This face-to-face conversation gave him the confidence to include our center as a collaborative site for pediatric JAK inhibitor clinical trials.
AI can help you find the “right person,” but only face-to-face interaction allows you to judge whether “this person is worth collaborating with”—their professional competence, work ethic, communication style—these are insights AI cannot convey. At conferences like ECCO, you can engage in deep conversations with dozens of peers in a short time, rapidly building trust in a way online communication simply cannot match.
4.3.3 Finally, AI can provide answers, but it cannot convey the “human warmth of compassion”
IBD treatment is not just science; it’s deeply human. We care for real patients whose pain, hope, and fears demand our compassionate understanding. This “human warmth” can only be conveyed through face-to-face interaction.
At ECCO’s Patient Representative Forum, I met a woman who had lived with Crohn’s disease for 20 years. She shared her journey from “despair” to “rebirth,” recounting the loneliness during treatment, her fear of side effects, and her longing for a normal life.Her story deeply moved all the experts present. A pharmaceutical executive immediately pledged to prioritize “patient experience” in new drug development—such as creating smaller tablets and more convenient dosing methods.
If we only relied on AI to analyze patient case reports, we might focus solely on cold metrics like “disease activity indices” and “mucosal healing rates,” overlooking patients’ genuine emotions. Face-to-face interactions, however, allow us to intuitively grasp patients’ needs, making our research and treatments more “human-centered.”
At ECCO, I also met a young researcher who had just begun his career in IBD research and felt uncertain about his future.We chatted for 15 minutes in the hallway. I shared my career journey—including setbacks and opportunities—and offered concrete advice. Later, he emailed me saying this conversation had reaffirmed his direction. This kind of “mentor-to-mentee encouragement and guidance” is irreplaceable by AI—AI can provide knowledge, but it cannot convey emotion or warmth.
In the AI era, we easily fall into “data worship,” believing that sufficient data and algorithms alone can solve every problem. Yet IBD treatment and research remain fundamentally a “human endeavor”—requiring mutual understanding, trust, and support among clinicians, researchers, pharmaceutical companies, and patients. Offline conferences like ECCO provide precisely the platform for these “human connections.”
Therefore, I firmly believe attending ECCO is not a “waste of time and money,” but a “long-term investment” in one’s career. Here, we experience intellectual exchanges, trust-building, and human warmth that AI cannot replicate—these are the core drivers propelling us forward. In the future AI era, the format of in-person conferences may evolve (such as incorporating hybrid online-offline elements), but the core value of “face-to-face interaction” will never become obsolete.
5. Closing Remarks: Call to Action for ECCO 2026 – Integrate Learnings with BIO International Convention 2026’s Global Biotech Momentum

5.1 Conferences as Catalysts: How ECCO Reshapes Your Career Path?
Reflecting on this in-depth exploration of ECCO 2026, it becomes increasingly clear: this conference is never merely a “one-way knowledge transfer,” but a true “catalyst” for advancing your professional growth. It isn’t like a thick textbook piling on theories; rather, it’s a partner walking alongside you, using cutting-edge technology, precise networking, and practical skills to help you break through career bottlenecks and discover new paths for growth.
I’ve witnessed countless peers achieve career transformations through ECCO: A community physician in suburban Chicago learned real-world application techniques for JAK inhibitors at the conference. Upon returning, they optimized their clinic’s treatment protocols for refractory IBD, significantly boosting patient satisfaction and earning an invitation to present at a local hospital symposium.A recent researcher connected with industry KOLs in the poster session, securing an opportunity to join a multicenter trial and publishing their first high-impact SCI paper within two years; a pharmaceutical product manager, through deep discussions with clinicians, pinpointed unmet patient needs and spearheaded a strategic pivot in the development of an oral small-molecule drug, making it more clinically relevant.
For me personally, ECCO’s catalytic effect has been equally profound. When I first attended a decade ago, I was merely a clinician focused solely on patient care, with little understanding of cutting-edge research or international collaboration. Yet through repeated participation, I encountered early breakthroughs in immunotherapy and connected with global leaders in stem cell research. This gradually shifted my practice from “purely treating patients” to a multifaceted model integrating “clinical work + research + international collaboration.”Today, I not only provide patients with cutting-edge treatment options but also advance the field through multicenter research—all stemming from the “connections” and “insights” ECCO has provided.
ECCO’s catalytic effect manifests in three key dimensions:
- It is a catalyst for knowledge renewal: Within three days, you gain exposure to 1-2 years’ worth of core advancements in global IBD research, eliminating the need to spend extensive time sifting through scattered literature and enabling you to swiftly keep pace with technological transformations.
- It catalyzes skill enhancement: From topic selection and efficient networking to post-conference implementation, you gain not only academic knowledge but also actionable practical skills that will make you more competitive in clinical practice, research, or industry work.
- It is a catalyst for network building: This gathering unites all key players in the global IBD ecosystem—clinicians, researchers, pharmaceutical executives, regulatory representatives, and patient advocates. The professional connections forged in a single conference may become a vital foundation for your career development over the next 5-10 years.
More importantly, ECCO catalyzes a shift in mindset—from “focusing on your niche” to “seeing the global ecosystem,” from “passively adopting technology” to “actively driving change,” and from “viewing IBD as a chronic condition” to “believing in the possibility of cure.” This transformative shift in thinking holds greater value than any specific knowledge or skill, propelling you further and more confidently along your career path.
5.2 Registration Sprint: 3 Steps to Easily Unlock ECCO 2026
If ECCO2026’s value has already resonated with you, the most important step now is to take action—the registration process is actually quite straightforward. I’ve outlined a “1-2-3 Step Guide” that will help you complete it effortlessly and avoid last-minute chaos:
5.2.1 Step 1: Complete Official Registration (Act Now—Early Bird Discount Countdown)
- Registration Channel: Go directly to the ECCO official website ([www.ecco-ibd.eu](www.ecco-ibd.eu)), locate the “ECCO2026 Congress” registration portal, and select your attendance type (In-Person/Online/Student—students receive significant discounts).
- Key Registration Points: When filling in personal details, accurately specify your professional field (e.g., “IBD Clinical Practice,” “Translational Research”) and affiliated institution. This helps match you with like-minded peers on the ECCO App. Payment options include credit cards and bank transfers. U.S. attendees may use health insurance or institutional research funds for payment. Remember to retain payment receipts for reimbursement purposes.
- Quick Tip: Early bird registration typically closes about 3 months before the conference. Registering early saves 20-30% and allows priority selection of your conference kit pickup time, avoiding on-site queues.
5.2.2 Step Two: Book Flights + Accommodation (Balancing Convenience and Value)
- Flights: Berlin has two main airports (Tegel TXL and Schönefeld SXF). Direct flights are available from the U.S. (Chicago, New York, Los Angeles all offer direct routes to Berlin). Booking 6-8 weeks in advance yields the lowest fares.For cost savings, consider connecting flights (e.g., via Frankfurt or Amsterdam), but ensure sufficient layover time to avoid missed connections.
- Hotels: Prioritize accommodations near the Berlin Exhibition Center (Messe Berlin) or with excellent transport links (e.g., close to U2 subway or S-Bahn lines). Ideally, the venue should be within a 20-minute walk or subway ride—daily commutes during the conference require minimal travel time to maximize focus on participation.Opt for hotels that include breakfast, as European hotel breakfasts are typically substantial and provide essential energy for a day of intensive meetings. Booking 4-6 weeks in advance secures better rates and allows you to select your preferred room location.
5.2.3 Step 3: Pre-Conference Preparation (Double Your Efficiency by Preparing Ahead)
- Download the ECCO App: After registration, immediately download the official app. Log in to sync your registration details, start filtering sessions, mark Category A meetings (refer to the session filtering method above), and set meeting reminders.
- Review Pre-Release Abstracts: The official website publishes pre-registration abstracts for all sessions 1-2 months before the conference. Spend 2-3 hours skimming through them, highlighting key data and speaker information for Category A sessions. Prepare questions in advance (use the Notion template for note-taking).
- Connect with target contacts: Proactively send private messages on LinkedIn to KOLs you wish to connect with (refer to the social strategy template above) to schedule meeting times during the event. If colleagues or peers are attending, create a group chat beforehand to share schedules and resources.
To help you check off items clearly, I’ve compiled a simple registration checklist for direct reference:
| Preparation Item | Completion Status (✓/×) | Notes |
| Complete official ECCO2026 registration | Confirm attendance type and payment status, save registration confirmation | |
| Book round-trip flights to Berlin | Record flight number and arrival time, allowing buffer time for transfers | |
| Book a hotel near the conference venue | Confirm check-in time, breakfast inclusion, and transportation convenience | |
| Download the ECCO App and sync your information | Mark Category A sessions and set reminders | |
| Review pre-release abstracts and prepare questions | Organize into a Notion template or notebook | |
| Send pre-conference private messages to target KOLs | Clearly state your communication intent and preferred time; save screenshots as backup | |
| Prepare conference materials (business cards, tablet/notebook, charger) | Print approximately 100 business cards; download offline materials to tablet in advance | |
| Apply for a visa (for non-EU countries) | Apply 3 months in advance; prepare conference proof, itinerary, and other required documents |
5.3 Vision Motivation: Become part of the IBD cure revolution
Finally, I’d like to share a phrase from ECCO 2026 that has stayed with me: “Curing IBD is never the achievement of any single individual, but the collective effort of every participant.”
Every technological breakthrough we discuss today—the resistance-breaking innovations of monoclonal antibody 2.0, the accessibility revolution of JAK inhibitors, the gut-repairing miracles of stem cell therapy—did not emerge from thin air. They are the culmination of countless experiments, exchanges, and case studies by clinicians, researchers, and pharmaceutical professionals. ECCO serves as the platform where these “individual efforts” converge into “collective strength.”
You may feel that as an ordinary clinician or a researcher just starting out, your impact is limited. But let me tell you: every conference you attend, every question you ask, every collaboration you initiate propels the field of IBD forward.A new technique you apply in clinical practice might free a patient from the burden of disease. A small data point you uncover in research could provide a crucial clue for a cure. A collaboration you establish with a colleague might accelerate the progress of a breakthrough study.
Just like Sarah, who regained a normal life through stem cell therapy; like Mike, who escaped the torment of frequent medication changes thanks to monoclonal antibody 2.0; like Emma, who avoided ostomy surgery through multidisciplinary teamwork—the hope for these patients stems from the dedication and persistence of every one of us in this field.
ECCO 2026 marks a watershed moment for IBD and a new beginning for each of us. It equips us with cutting-edge technologies, precise professional networks, and practical skills. Now, we must bring these gains back to our work—transforming knowledge into action, connections into collaboration, and dreams into reality.
Over the next 5-10 years, IBD will evolve from “chronic management” to “biological cure”—not a distant dream, but a reality we will collectively witness and advance. You are an indispensable part of this healing revolution.
So don’t hesitate—complete your registration now, prepare yourself, and head to Berlin. There, you’ll meet like-minded peers, spark intellectual exchanges, discover your professional path, and become a force for changing the lives of IBD patients.
I’ll be waiting for you at ECCO 2026. Together, we’ll witness the dawn of cure and become better versions of ourselves.







