1. Conference Background and Strategic Positioning: A Critical Turning Point in the Development of Innovative Drugs in China for Bio International 2025
In the first quarter of 2025, the total value of license-out transactions for innovative drugs in China reached US$36.929 billion, accounting for 70% of the total transaction value for the whole of 2024. The upfront payment of US$900 million far exceeded the total amount of financing in the primary market during the same period.

This milestone figure signifies that domestically developed innovative drugs have officially entered a new phase of development, transitioning from capital-driven growth to technology commercialization.
Against the backdrop of accelerating restructuring in the global biopharmaceutical landscape, the industry faces three major challenges: international competition, capital allocation efficiency, and technology commercialization.

- How to capitalize on the “window of opportunity” on the global stage?
- How to establish seamless collaboration across the entire ecosystem?
- How to overcome the “last mile” in product commercialization?
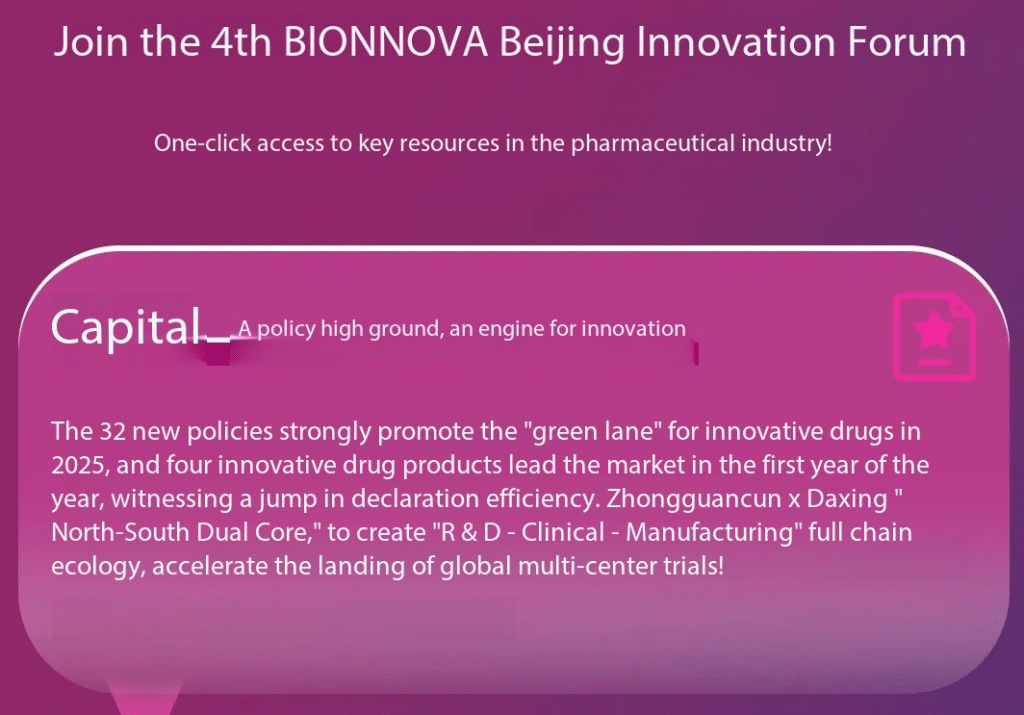
It is precisely at this historic moment that the 4th BIONNOVA Beijing Innovation Forum will be held on September 23-24, 2025, at the Langrizi West Hill Garden Hotel in Beijing.

This year’s forum, themed “Gathering Momentum, Breaking Free from the Cocoon,” is supported by leading domestic and international pharmaceutical companies, including Pfizer, Novo Nordisk, Shijiazhuang Pharmaceutical Group, Legend Biotech, Zai Lab, and Tencent BioPharma. It focuses on full-chain innovation collaboration and clinical needs alignment, bringing together key players across the pharmaceutical industry value chain to create a practical, action-oriented industry exchange platform.
1.1 Beijing: A Strategic Stronghold for Pharmaceutical Innovation in China
The choice of Beijing as the venue for the forum has far-reaching strategic significance. As a core pillar of the national innovative drug strategy, Beijing has always played multiple roles in policy formulation, technological leadership, and talent aggregation:
- Policy Innovation:
- The “32 New Policies” introduced at the beginning of 2025 have provided strong support, promoting an “express lane for innovative drugs”. By the start of the year, four innovative drugs had already been launched, leading the way and demonstrating a significant improvement in application efficiency.
- Ecosystem Development:
- Zhongguancun and Daxing have formed a “north-south dual core” structure, creating a comprehensive ecosystem that spans research and development, clinical trials, and manufacturing, thereby accelerating the implementation of global multi-center trials.
- Talent Aggregation:
- Leveraging Beijing’s abundant research institutions (such as the National Center for Nanoscience and Technology and Tsinghua University) and clinical research organizations, a talent aggregation effect has been formed.
As a policy hub and innovation engine, Beijing is continuously fostering a momentum for pharmaceutical innovation that influences the entire nation, providing domestic and international companies with a unique innovation ecosystem.
2. Core Agenda and Cutting-Edge Topics: Comprehensive Coverage of the Entire Pharmaceutical Innovation Value Chain at bio international 2025
2.1 Forum Structure and Content Design
The 4th BIONNOVA Beijing Innovation Forum has been carefully designed with 1 main forum and 16 specialized sub-forums, covering over 140 cutting-edge topics, forming a high-density topic matrix that spans the entire process from project initiation to commercialization. This structural design ensures the breadth and depth of content, catering to the needs of participants with diverse professional backgrounds.
Overview of Sub-Forum Arrangements:
| Forum Name | Date | Key Points |
| Plenary Session: Full-chain Ecosystem Collaboration | September 23 | Interpretation of industrial policies, analysis of global trends, and ecological synergy models |
| Antibody New Drug Discovery and Design | September 23 | Frontier directions such as TCE dual-antibody/triple-antibody and dual-payload ADCs |
| ADC Development and Commercialization Strategies | September 23 | ADC drug process transfer and commercialization production strategies |
| Immune Cell and Stem Cell Therapy | September 23 | Progress in the clinical application of universal CAR-T and stem cells |
| Small Molecule Anticancer Drugs Focus | September 23 | Target innovation, combination therapy, and drug resistance mechanism research |
| Small Nucleic Acids and mRNA Focus | September 23 | mRNA vaccine development and nucleic acid drug production processes |
| New Drug Registration and Filing | September 23 | China-US dual reporting strategies and international multi-center trial design |
2.2 In-depth Focus on Cutting-Edge Technology Fields
2.2.1 Antibody Drugs: Leading the Revolution in Engineered Antibody Technology
China has risen to the forefront of the global engineered antibody technology field. This forum will focus on:
- TCE Bispecific/Trispecific Antibodies:
- Exploring differentiated innovation pathways in molecular design and discussing how to overcome affinity balance challenges in bispecific antibody development
- Dual-payload ADCs:
- Discussing breakthroughs in linker technology and payload combination strategies for next-generation antibody-drug conjugates
- Cost Reduction and Efficiency Improvement in Industrialization:
- Yan Xu (Director of the Biotechnology Center at Chutian Technology) will share strategies for cost reduction and efficiency improvement in the industrialization of antibody drugs
- CMC Analysis and Control:
- Liu Mingsong (Executive Director of R&D at China Resources Bio) will analyze CMC analysis and control strategies in biopharmaceuticals and related defect cases
2.2.2 Cell and Gene Therapy (CGT): The Industrialization Path of Breakthrough Therapies
The CGT field continues to make key progress in areas such as universal CAR-T, stem cells, and gene editing. The forum will delve into:
- Quality Control Challenges:
- Xu Guodong (R&D Director, Wuhan Jiachuang Bio) will share “Quality Control and Method Development for Cell Therapy Products”, based on technical achievements from China-US-EU regulatory submission strategies
- Production Process Bottlenecks:
- Focusing on solutions to core production challenges, such as closed-loop automated production systems and large-scale production of viral vectors
- Accelerating Clinical Translation:
- Shi Hui (Managing Partner at Chengnuo Medicine) will present on “Clinical Research Progress in Stem Cell Therapy for Stroke.”
- Regulatory Science Advances:
- Tan Yi (Vice President and Technical Director at Qilu Cell) will analyze “Clinical Research Pathway Selection for Cell Products Under the Current Regulatory Framework.”
2.2.3 Small Molecule Drugs: The Modern Revival of a Century-Old Classic
In the era of precision medicine, small-molecule drugs have demonstrated unique potential. This forum has specially set up a “Small Molecules & Complex Formulations & Quality Control” thematic module:
- Breakthroughs in Tumor Small Molecule Drugs:
- Focusing on target innovation (such as FGFR4, KAT6, etc.), combination therapy, and resistance mechanism research
- Advanced Formulation Technology:
- Exploring the advantages and applications of novel formulation technologies in the development of improved new drugs, such as innovative mechanisms for tumor penetration via nanotechnology
- Representative Case Studies:
- Beta Pharmaceuticals:
- The successful experience of Kamena, China’s first domestically developed small molecule targeted anticancer drug
- Huahao Zhongtian:
- The R&D journey of Yutidelong Injection (a new microtubule inhibitor molecule), a National Class 1 New Drug
- Yudao Bio:
- AI-enabled development strategy for p53 Y220C allosteric reactivators
- Beta Pharmaceuticals:
- CMC Quality Control Upgrades:
- Li Wenqi (Director of the Protein Research Technology Center at Tsinghua University) will discuss quality control and analytical techniques for biopharmaceuticals
2.2.4 Nucleic Acid and Peptide Drugs: The Emerging TIDES Therapy
Nucleic acid and peptide drugs are experiencing breakthroughs in indication expansion, prolonged efficacy, and delivery technology:
- mRNA Technology Evolution:
- Focusing on sequence optimization, delivery system innovation, and freeze-drying processes in the development of next-generation mRNA vaccines
- Peptide Drug Development:
- MomiSt, a small-molecule PDE4 inhibitor independently developed by Hemei Pharmaceutical, will be shared as a case study
- Industrialization Challenges:
- Discussing the application of large-scale synthesis, purification processes, and freeze-drying technology in nucleic acid drug production
- Breakthroughs in Delivery Technology:
- Xu Yongyu, Market Development Manager at Rui Fu Di, will share insights on “Frontier Applications in Life Sciences – Gene Delivery Special Topic”
2.3 Clinical Trials and Regulatory Filings: Bridging the Final Gap to Market Launch
Leveraging Beijing’s policy advantages, the forum will delve into:
- International Multi-Center Trial Design:
- Huang Wei (Chief Medical Officer for Oncology at Shijiazhuang Pharmaceutical Group) will share insights on “Considerations and Case Studies in Global Synchronized Clinical Development.”
- China-US Dual Filing Strategy:
- Kong Weiqing, Project Leader of the Non-Clinical Science Department at Zhaoyan New Drug, will analyze “Key Considerations in Non-Clinical Safety Evaluation of Antidiabetic Drugs Using DART.”
- Accelerated Approval Pathways:
- Focusing on the synergistic strategies between the China National Medical Products Administration’s (NMPA) breakthrough therapy designation criteria and the FDA’s Fast Track program
- Real-World Evidence (RWE) Application:
- Exploring how to utilize RWE to support post-marketing studies and indication expansion for drugs
3 Conference Value and Unique Platform: Building a Global Pharmaceutical Innovation Cooperation Network for Bio International 2025

3.1 Resource Matching System: A Closed Loop from Technology to Capital
- BIONNOVA ONE-TO-ONE Customized Negotiations:
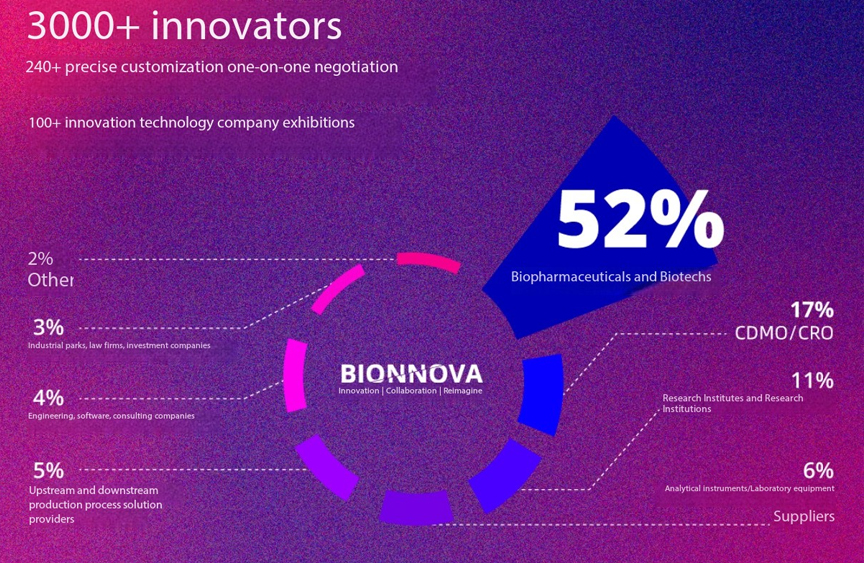
- Over 240 customized one-on-one negotiations will be conducted on-site, covering key areas such as technology transfer, pipeline collaboration, and investment and financing. This highly acclaimed signature service helps participating companies quickly identify the most valuable business partners, create real collaboration scenarios, and precisely match demand with supply.
- Innovation Technology Exhibition Area:
- Over 100 innovative exhibitors will showcase their technologies, including cutting-edge innovations, smart production platforms, and industry services, providing a centralized platform for direct engagement with industry leaders. Past exhibitors include leading companies such as Zhaoyan New Drug (Booth B12), Wuhan Jiachuang Biotechnology (Booth A03), and Moji Biotechnology (Booth E03).
- Talent and Knowledge Services:
- Specialized services such as supply chain consulting, talent consulting, intellectual property consulting, information consulting, project evaluation, and legal consulting are provided to address the supporting needs throughout the entire innovation cycle of enterprises.

3.2 International Platform Value
For international exhibitors and attendees, the BIONNOVA Beijing Innovation Forum offers a unique platform value:
- China Market Access Gateway:
- The forum provides in-depth analysis of China’s pharmaceutical regulatory policy reforms (such as the new version of the Drug Registration Management Measures), helping international companies seize the critical window of opportunity for market access in China. The 2025 Beijing “32 New Policies” provide an accelerated approval pathway for imported innovative drugs.
- Localization Collaboration Hub:
- With a network of over 3,000 industry decision-makers, including executives from multinational pharmaceutical companies, leaders of top pharmaceutical firms, biotech founders, CXOs, research experts, and capital industry leaders, the forum enables participants to connect with China’s core pharmaceutical industry circles within two days.
- Technology Introduction and Export Platform:
- The forum showcases China’s domestic innovative achievements (such as breakthroughs in CAR-T, ADC, and PD-1 fields) while introducing international cutting-edge technologies (such as Emulate’s organ-on-a-chip technology), promoting cross-border technology transfer and collaborative development.
3.3 Industry-Academia-Research Integration and Transformation
The forum places particular emphasis on the integration of basic research and industrial applications:
- Frontier Research Presentations:
- Professor Wang Yonghui’s team from Fudan University will share research findings on novel A2AR/A2BR dual-target small-molecule antagonists, demonstrating the conversion pathway from basic research to drug discovery.
- Organoid Technology Applications:
- Luo Haiqing, General Manager of Moji Bio, will introduce the application of the “organoid culture multifunctional toolkit” in drug screening.
- AI-Driven R&D:
- Yudao Bio will demonstrate the application of generative AI technology in the generation of novel small molecule structures, breaking through the chemical space limitations of traditional drug discovery.
- Organ-on-a-Chip Technology:
- Emulate will share strategies for using organ-on-a-chip technology in drug hepatotoxicity assessment, providing preclinical evaluation models that more closely mimic human physiology.
4 Professional Attendee Guide: Maximizing Your Forum Value at Bio International 2025

4.1 Registration and Ticket Information
- Early Bird Discount:
- Register by August 31, 2025, to enjoy a 15% discount.
- Free Attendance Opportunities:
- The forum offers 100 free seats (limited to pharmaceutical companies, investment institutions, and research institutes), subject to approval.
- Group Registration:
- Groups of five or more can enjoy additional discounts.
- Registration Channels:
- Official Website: www.bionnovacon.com/beijing2025
- WeChat Official Account: BIONNOVA Xiaodeng (offers 1-on-1 customized services)
4.2 Visa and Travel Recommendations
- Visa Application:
- International participants may apply for an M-type business visa; the organizing committee will provide an official invitation letter for support
- Optimal Arrival Time:
- It is recommended to arrive in Beijing two days in advance (September 21) to adjust to the time zone and participate in pre-conference activities
- Hotel Reservations:
- Forum Main Hotel:
- Beijing Langrizi West Hill Garden Hotel (special rates available)
- Recommended Hotels:
- Beijing Shangri-La Hotel, Beijing Marriott Hotel (forum shuttle service available)
- Forum Main Hotel:
4.3 Efficient Participation Strategies
To help participants maximize the value of the forum, we recommend:
- Pre-Conference Preparation:
- Download the BIONNOVA official app, review the agenda in advance, and mark must-attend sessions
- Schedule ONE-TO-ONE meeting slots (bookings open 3 weeks in advance)
- Prepare sufficient business cards and company profile materials (in both Chinese and English)
- On-site strategies:
- Theme selection:
- Based on professional needs, we recommend focusing on 1-2 vertical fields for in-depth participation (e.g., focusing on ADC or CGT)
- Networking focus:
- Make full use of informal networking opportunities during breaks and lunches to build connections
- Exhibition area guide:
- Research the list of exhibitors in advance and mark must-visit booths (e.g., Zhaoyan New Drug B12, Jiachuang Bio A03, etc.)
- Theme selection:
- Post-Event Follow-Up:
- Complete follow-up with new contacts within 48 hours after the forum concludes
- Monitor official meeting summaries and presentation materials released by BIONNOVA (exclusive to registered attendees)
- Mark the 2026 conference date (typically late September)
4.4 Beijing Business and Cultural Exchange
During the forum, attendees can experience Beijing’s unique business and cultural life:

- Professional visits:
- Zhongguancun Life Science Park:
- China’s largest biopharmaceutical industry cluster in the north
- Beijing Economic and Technological Development Zone:
- Home to R&D centers of multinational pharmaceutical companies such as Bayer and Sanofi
- National Center for Nanoscience and Technology:
- Learn about China’s cutting-edge research in nanomedicine
- Cultural experiences:
- Forbidden City Museum:
- Approximately a 40-minute drive from the forum hotel, offering a glimpse into the beauty of traditional Chinese architecture
- Fragrant Hills Park:
- A scenic spot near the Forum Hotel, renowned for its autumn foliage
- Beijing Cuisine:
- Recommended dishes include Beijing roast duck (Quanjude, Da Dong), traditional Beijing-style fried noodles with soybean paste, and imperial court pastries
- Business Hospitality:
- High-end business banquets recommended:
- Ran Sushi (Qiaofu Fangcao Di), Da Dong (Workers’ Stadium Branch)
- Specialty business gifts:
- Tongrentang health products, Forbidden City cultural and creative items, and Beijing embroidery crafts
- Forbidden City Museum:
- Zhongguancun Life Science Park:
5 Review of the scale and participation of the 2024 forum
5.1 Core data comparison
| Indicators | 2024 (3rd edition) | 2025 (4th edition)Expected | Growth trend |
| Number of participants | 3,127 professional visitors | 3,000+ decision makers | Stable (base number saturated) |
| Number of exhibiting companies | 68 partners and sponsors | 100+ innovative technology companies | 147% |
| One-on-one meetings | 253 targeted matchmaking sessions | 240+ customized negotiations | Optimized matching efficiency |
| Forum sessions and topics | 11 parallel forums | 17 special forums + 140+ topics | 155% Issue density |
| Covered tracks | 8 major areas including antibodies, CGT, small molecules, nucleic acids, etc | New additions include AI pharmaceuticals, organoids, complex formulations, etc | Expansion of technological frontiers |
5.2 International Participation Analysis
- Regional Distribution:
- In 2024, 65% of exhibitors will come from the North China region (Beijing/Tianjin/Hebei), 20% from the Yangtze River Delta (Shanghai/Jiangsu), and 15% from international exhibitors (Europe/America/Japan/Korea).
- Participation of Leading Companies:
- International pharmaceutical companies such as Pfizer, Shijiazhuang Pharmaceutical Group, and Zhaoyan New Drug (Booth B12), as well as leading CROs, will exhibit or present. Zhaoyan New Drug will share a case study on “Non-clinical Safety Evaluation of Antidiabetic Drugs.”
- Negotiation and Conversion Outcomes:
- In 2024, 21 technical cooperation agreements were signed on-site, covering topics such as antibody process optimization and CGT production solutions, with an average success rate of 8.3%.
5.3 Factors driving scale expansion
- Policy benefits:
- Beijing’s “32 new policies” are accelerating the market launch of innovative drugs (four new drugs have been approved in Q1 2025), attracting international pharmaceutical companies to increase their investment in the North China market.
- Industrial chain synergy upgrade:
- The 2025 forum introduced new topics on the “clinical-to-commercialization closed-loop process” (such as international compliance pathways and license-out strategies), addressing key pain points for international pharmaceutical companies entering China.
- Resource aggregation effect:
- The positive reputation from previous editions has driven leading capital firms (such as Sequoia Capital and Hillhouse Capital) and multinational corporations (such as Novo Nordisk and Zai Lab) to continue increasing their sponsorship.
5.4 Recommendations for International Exhibitors
- Priority Participation Areas:
- Focus on CGT process development (35% of exhibitor demand), ADC internationalization strategies (new forum in 2025), and AI-driven drug discovery (an extension of the popular topic from the 2024 Shanghai event to Beijing).
- Secure Scarce Resources:
- Over 240 one-on-one meeting slots for 2025 are now open for booking. It is recommended to submit a list of technical collaboration/pipeline needs in advance to match with local biotech companies.
Key Conclusions:
The BIONNOVA Beijing Forum is evolving from a regional conference into a core international platform for North China. In 2024, it will solidify the industrial foundation, and in 2025, it will focus on ecosystem closure. International exhibitors can leverage policies and clinical resources to break through the “last mile” of the Chinese market.
6 Conclusion: Collaborating to Create the Future of Global Pharmaceutical Innovation in Bio International 2025
- The 4th BIONNOVA Beijing Innovation Forum stands at a critical turning point for China’s pharmaceutical innovation, themed “Gathering Momentum, Transforming into a Butterfly,” providing a rare platform for global pharmaceutical innovators to exchange ideas. The forum not only showcases China’s latest achievements in antibody drugs, cell therapy, and small-molecule innovative drugs but also aims to build an ecosystem connecting global innovative resources.
- For international participants, this is not only a window into China’s pharmaceutical market but also a strategic opportunity to engage in the internationalization of China’s innovative drugs. From the explosive growth of license-out transactions to deep involvement in international multi-center clinical trials and innovative models of cross-border technological cooperation, China’s pharmaceutical innovation is integrating into the global innovation network with unprecedented openness.
- On September 23-24, 2025, let us gather at the Langri West Hill Garden Hotel in Beijing to participate in this pharmaceutical innovation event rooted in North China and radiating globally. Together, we will seize the transformative development opportunities for China’s innovative drugs as they transition from “survival adaptation” to “leading change,” and jointly usher in the next golden decade of global pharmaceutical innovation!